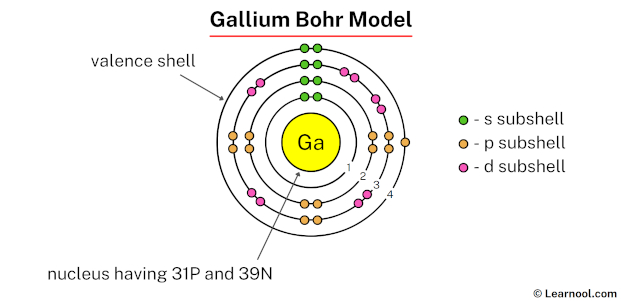
In the gallium Bohr model, the nucleus is composed of 31 protons and 39 neutrons. Surrounding this nucleus are four electron shells, accommodating a total of 31 electrons.
To draw the gallium Bohr model, represent the 31 protons, 39 neutrons, and 31 electrons. Begin by sketching the nucleus, and then draw the four electron shells. The first three shells should contain 2, 8, and 18 electrons, respectively, while the fourth shell holds the remaining 3 electrons.
Steps
Find protons, neutrons, and electrons of gallium atom
Gallium has 31 protons, 39 neutrons, and 31 electrons.
Gallium protons
- Protons = atomic number
From the periodic table, find the atomic number of gallium.
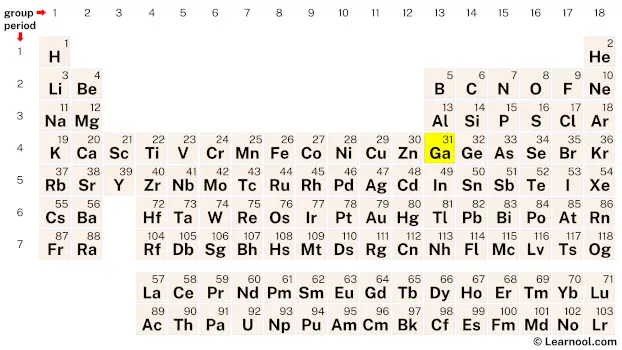
The atomic number of gallium is 31. Hence, gallium has a total of 31 protons.
Gallium neutrons
- Neutrons = atomic mass – atomic number
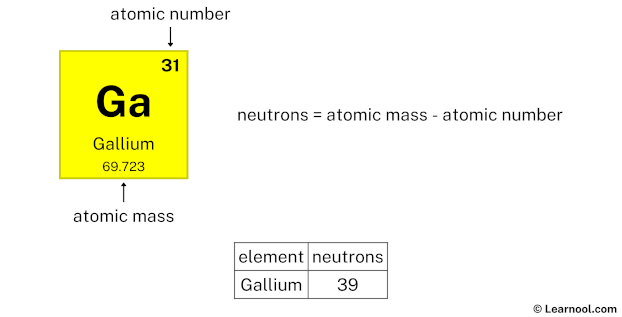
The atomic mass of gallium is 69.723, so we’ll take the roundup value as 70. And the atomic number of gallium is 31.
Subtract the atomic number (31) from the atomic mass (70). Hence, gallium has a total of 70 – 31 = 39 neutrons.
Gallium electrons
- Electrons = atomic number
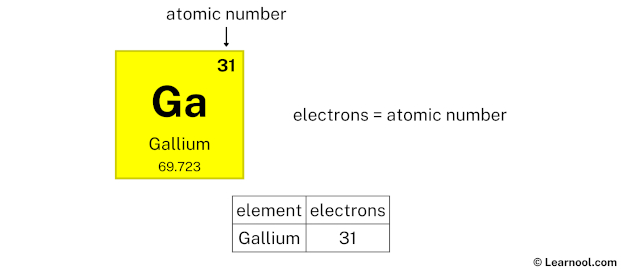
The atomic number of gallium is 31. Hence, gallium has a total of 31 electrons.
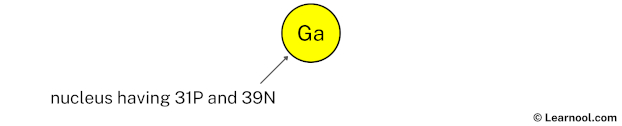
Draw nucleus of gallium atom
The nucleus of a gallium atom contains 31 protons and 31 neutrons. So draw the nucleus of gallium atom as follows:
Now in the next step, draw the 1st electron shell and start marking electrons.
Draw 1st electron shell
Remember that we have a total of 31 electrons.
The 1st electron shell (containing s subshell) can hold up to a maximum of 2 electrons. So draw the 1st electron shell as follows:
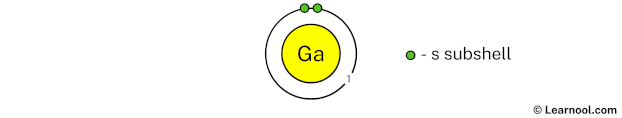
In the above image, 1 represents the 1st electron shell that contains 1s subshell. And the green color represents the number of electrons in that subshell. This means that the 1st electron shell has a total of 2 electrons.
Since we have already used 2 electrons in the 1st electron shell, now we have 31 – 2 = 29 electrons left. So in the next step, we have to draw the 2nd electron shell.
Draw 2nd electron shell
The 2nd electron shell (containing s subshell and p subshell) can hold up to a maximum of 8 electrons. So draw the 2nd electron shell as follows:
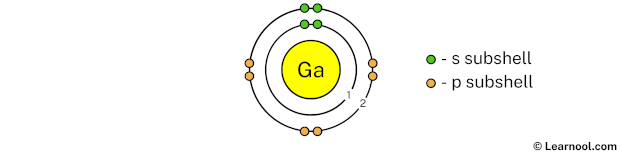
In the above image, 2 represents the 2nd electron shell that contains 2s and 2p subshells. And the green and orange color represents the number of electrons in that subshell. This means that the 2nd electron shell has a total of 8 electrons.
Now we have already used 10 electrons in 1st and 2nd electron shells, so we have 31 – 10 = 21 electrons left. So in the next step, we have to draw the 3rd electron shell.
Draw 3rd electron shell
The 3rd electron shell (containing s subshell, p subshell, and d subshell) can hold up to a maximum of 18 electrons. So draw the 3rd electron shell as follows:
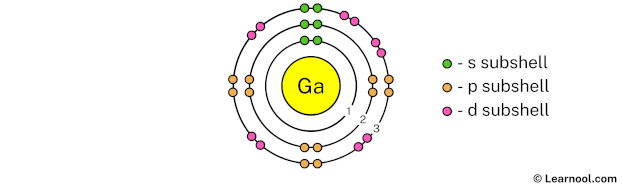
In the above image, 3 represents the 3rd electron shell that contains 3s, 3p and 3d subshells. And the green, orange, and pink color represents the number of electrons in that subshell. This means that the 3rd electron shell has a total of 18 electrons.
Now we have already used 28 electrons in 1st, 2nd, and 3rd electron shells, so we have 31 – 28 = 3 electrons left. So in the next step, we have to draw the 4th electron shell.
Draw 4th electron shell
The 4th electron shell (containing s subshell, p subshell, d subshell, and f subshell) can hold up to a maximum of 32 electrons. So draw the 4th electron shell as follows:
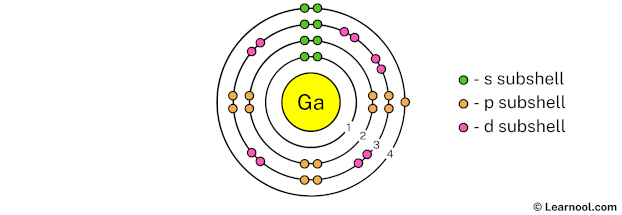
In the above image, 4 represents the 4th electron shell that contains 4s and 4p subshells. And the green and orange color represents the number of electrons in that subshell. This means that the 4th electron shell has a total of 3 electrons.
That’s it! This is the final Bohr model of gallium atom as we have used all 31 electrons: 2 electrons in the 1st electron shell, 8 electrons in the 2nd electron shell, 18 electrons in the 3rd electron shell, and 3 electrons in the 4th electron shell.
Next: Germanium Bohr model
Related
More topics
External links
- File:31 galium (Ga) enhanced Bohr model.png – Wikimedia Commons
- Chemical Elements.com – Gallium (Ga) – Chemical Elements.com
- What is the Bohr model for Gallium? – Topblogtenz
- Gallium (Ga) – Periodic Table – ChemicalAid
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
