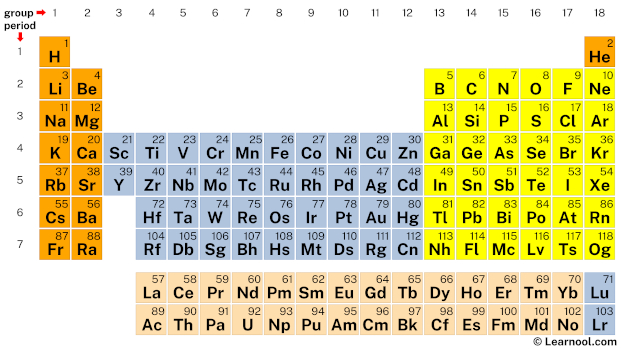
The periodic table, commonly referred to as the periodic table of elements, presents all chemical elements in a tabular form. It consists of 118 elements arranged by ascending atomic numbers, with the table comprising the s-block, p-block, d-block, and f-block. Its eighteen vertical columns are known as groups, while the seven horizontal rows are termed periods.
The periodic table is widely used in physics, chemistry, and various other sciences as a visual guide to the fundamental principles of chemistry. It illustrates the periodic law, which states that the properties of chemical elements follow a recurring pattern based on their atomic numbers. Dmitri Mendeleev, a Russian chemist, introduced this law, connecting chemical properties to atomic mass. Despite gaps in Mendeleev’s table due to undiscovered elements, he successfully predicted properties using the periodic law.
The periodic table is dynamic and continues to evolve with scientific advancements. Elements with atomic numbers up to 94 are naturally occurring, while those beyond must be artificially created or synthesized in a laboratory setting. Although the first seven rows of the periodic table now encompass all 118 initial elements, ongoing chemical characterization of the heaviest elements is crucial to ensure that their properties align with their respective positions on the table.
Interactive periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – s block | – p block | ||
| – d block | – f block | ||
Classification
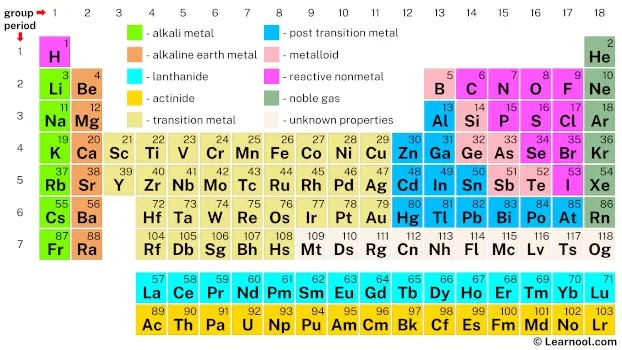
There are many terms used to describe groups of elements that behave similarly. Alkali metal, alkaline earth metal, pnictogen, chalcogen, halogen, and noble gas are six of the group names recognized by the IUPAC.[1]
Although there is no official IUPAC definition of which elements are metals, nonmetals, or metalloids, the periodic table organizes elements into distinct groups, each with its unique characteristics. Alkali metals, for example, are known for their exceptional reactivity, ready to form alkaline solutions when they react with water. On the other hand, noble gases exhibit remarkable inertness and rarely participate in chemical reactions.
The terms “post-transition metal” and “poor metal” have been suggested as alternatives for categorizing certain metals that appear after the transition metals. These elements, positioned between the main-group and transition metals, often showcase unique properties, with zinc, cadmium, and mercury sometimes included in the post-transition metal category. However, despite these proposed terms, a consensus has not been reached within the scientific community. This ongoing debate highlights the challenge in appropriately classifying these metals.
The elements from La to Lu, collectively known as the lanthanides, share striking similarities. Originally, this group included elements from Ce to Lu, but over time, lanthanum was added due to its analogous properties. Lanthanides exhibit magnetic properties and find applications in technologies like magnetic resonance imaging (MRI). On the other hand, the elements from Ac to Lr (previously, Th to Lr) are referred to as actinides. Unlike the lanthanides, the actinides showcase a broader range of properties. Some actinides, such as uranium and plutonium, play crucial roles in nuclear technology, demonstrating their significance beyond their lanthanide counterparts. This diverse behavior among actinides reflects the broader variation within this group.
History
Carbon, sulfur, iron, copper, silver, tin, gold, mercury, and lead are some of the chemical elements known since ancient history. These elements exist in their natural state and were relatively easy to mine using primitive tools. Meanwhile, zinc, arsenic, antimony, and bismuth were among the elements recognized during the age of alchemy.
In 1817, Johann Wolfgang Döbereiner, a German physicist, made one of the first attempts to categorize the elements. By 1829, he discovered a method to organize certain elements into groups of three, where each group’s members shared similar properties. These groupings, termed “triads,” consisted of chlorine, bromine, and iodine; calcium, strontium, and barium; lithium, sodium, and potassium; and sulfur, selenium, and tellurium.[2]

Dmitri Mendeleev, a Russian chemist, made a pivotal discovery in the development of the periodic table. Mendeleev, who was deeply committed to refine and champion his framework, left an indelible mark on the scientific community. Despite contemporaneous discoveries by other chemists, including Meyer, who proposed alternative variations of the periodic system, Mendeleev’s systematic approach to organizing elements based on their atomic weights, initiated in February 1869, had the most significant impact.

His printed table first appeared in the publication of the Russian Chemical Society in May 1869, following Mendeleev’s settlement on an acceptable arrangement. Mendeleev openly acknowledged that instances where an element appeared to be missing from the system meant that the element had not yet been discovered. In 1871, he published a comprehensive article featuring an updated version of his table and publicly shared his predictions for undiscovered elements.
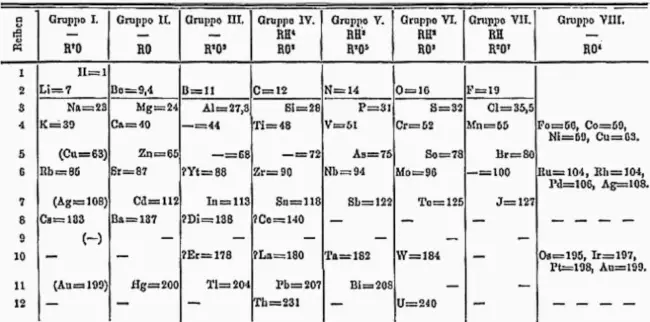
Once the periodic table was ultimately organized, the descriptive value of the relationship between elements was recognized. However, this appreciation wasn’t universal. In 1881, Mendeleev and Meyer engaged in a heated exchange of papers, published in the British newspaper Chemical News, where they debated the significance of the periodic table. These papers, not only from Mendeleev and Meyer but also from others, contained critiques of the idea of periodicity.
In 1882, the Royal Society in London honored Mendeleev and Meyer with the Davy Medal for their groundbreaking work in classifying the elements. Remarkably, two of Mendeleev’s predicted elements had already been discovered by that time. It is important to highlight that his predictions weren’t explicitly acknowledged in the justification for the award. Despite this lack of recognition for his predictions, by 1890, Mendeleev’s periodic table had gained widespread acceptance as a fundamental cornerstone of chemical knowledge.[3]
Elements list
Refer to the provided table for a concise presentation of all 118 chemical elements arranged in sequential order.
| Element 1: Hydrogen (H) | |
| Element 2: Helium (He) | |
| Element 3: Lithium (Li) | |
| Element 4: Beryllium (Be) | |
| Element 5: Boron (B) | |
| Element 6: Carbon (C) | |
| Element 7: Nitrogen (N) | |
| Element 8: Oxygen (O) | |
| Element 9: Fluorine (Fl) | |
| Element 10: Neon (Ne) | |
| Element 11: Sodium (Na) | |
| Element 12: Magnesium (Mg) | |
| Element 13: Aluminium (Al) | |
| Element 14: Silicon (Si) | |
| Element 15: Phosphorus (P) | |
| Element 16: Sulfur (S) | |
| Element 17: Chlorine (Cl) | |
| Element 18: Argon (Ar) | |
| Element 19: Potassium (K) | |
| Element 20: Calcium (Ca) | |
| Element 21: Scandium (Sc) | |
| Element 22: Titanium (Ti) | |
| Element 23: Vanadium (Va) | |
| Element 24: Chromium (Cr) | |
| Element 25: Manganese (Mg) | |
| Element 26: Iron (Fe) | |
| Element 27: Cobalt (Co) | |
| Element 28: Nickel (Ni) | |
| Element 29: Copper (Cu) | |
| Element 30: Zinc (Zn) | |
| Element 31: Gallium (Ga) | |
| Element 32: Germanium (Ge) | |
| Element 33: Arsenic (As) | |
| Element 34: Selenium (Se) | |
| Element 35: Bromine (Br) | |
| Element 36: Krypton (Kr) | |
| Element 37: Rubidium (Rb) | |
| Element 38: Strontium (Sr) | |
| Element 39: Yttrium (Yt) | |
| Element 40: Zirconium (Zr) | |
| Element 41: Niobium (Nb) | |
| Element 42: Molybdenum (Mo) | |
| Element 43: Technetium (Tc) | |
| Element 44: Ruthenium (Ru) | |
| Element 45: Rhodium (Rh) | |
| Element 46: Palladium (Pd) | |
| Element 47: Silver (Ag) | |
| Element 48: Cadmium (Cd) | |
| Element 49: Indium (In) | |
| Element 50: Tin (Sn) | |
| Element 51: Antimony (Sb) | |
| Element 52: Tellurium (Te) | |
| Element 53: Iodine (I) | |
| Element 54: Xenon (Xe) | |
| Element 55: Caesium (Cs) | |
| Element 56: Barium (Ba) | |
| Element 57: Lanthanum (La) | |
| Element 58: Cerium (Ce) | |
| Element 59: Praseodymium (Pr) | |
| Element 60: Neodymium (Nd) | |
| Element 61: Promethium (Pm) | |
| Element 62: Samarium (Sm) | |
| Element 63: Europium (Eu) | |
| Element 64: Gadolinium (Gd) | |
| Element 65: Terbium (Tb) | |
| Element 66: Dysprosium (Dy) | |
| Element 67: Holmium (Ho) | |
| Element 68: Erbium (Er) | |
| Element 69: Thulium (Tm) | |
| Element 70: Ytterbium (Yb) | |
| Element 71: Lutetium (Lu) | |
| Element 72: Hafnium (Hf) | |
| Element 73: Tantalum (Ta) | |
| Element 74: Tungsten (W) | |
| Element 75: Rhenium (Re) | |
| Element 76: Osmium (Os) | |
| Element 77: Iridium (Ir) | |
| Element 78: Platinum (Pt) | |
| Element 79: Gold (Au) | |
| Element 80: Mercury (Hg) | |
| Element 81: Thallium (Tl) | |
| Element 82: Lead (Pb) | |
| Element 83: Bismuth (Bi) | |
| Element 84: Polonium (Po) | |
| Element 85: Astatine (At) | |
| Element 86: Radon (Rn) | |
| Element 87: Francium (Fr) | |
| Element 88: Radium (Ra) | |
| Element 89: Actinium (Ac) | |
| Element 90: Thorium (Th) | |
| Element 91: Protactinium (Pa) | |
| Element 92: Uranium (U) | |
| Element 93: Neptunium (Np) | |
| Element 94: Plutonium (Pu) | |
| Element 95: Americium (Am) | |
| Element 96: Curium (Cm) | |
| Element 97: Berkelium (Bk) | |
| Element 98: Californium (Cf) | |
| Element 99: Einsteinium (Es) | |
| Element 100: Fermium (Fm) | |
| Element 101: Mendelevium (Md) | |
| Element 102: Nobelium (No) | |
| Element 103: Lawrencium (Lr) | |
| Element 104: Rutherfordium (Rf) | |
| Element 105: Dubnium (Db) | |
| Element 106: Seaborgium (Sg) | |
| Element 107: Bohrium (Bh) | |
| Element 108: Hassium (Hs) | |
| Element 109: Meitnerium (Mt) | |
| Element 110: Darmstadtium (Ds) | |
| Element 111: Roentgenium (Rg) | |
| Element 112: Copernicium (Cn) | |
| Element 113: Nihonium (Nh) | |
| Element 114: Flerovium (Fl) | |
| Element 115: Moscovium (Mc) | |
| Element 116: Livermorium (Lv) | |
| Element 117: Tennessine (Ts) | |
| Element 118: Oganesson (Og) | |
Related
- Periodic table
- Block (periodic table)
- Group (periodic table)
- Period (periodic table)
- Metal
- Alkali metal
- Alkaline earth metal
- Lanthanide
- Actinide
- Transition metal
- Post-transition metal
- Metalloid
- Nonmetal
- Reactive nonmetal
- Noble gas
References
- Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005) – IUPAC
- Development of the periodic table – Royal Society of Chemistry
- The Periodic Table: Its Story and Its Significance (pg 156-169) – Google Books
External links
- Periodic Table – Ptable
- Periodic Table of Elements – National Institutes of Health (.gov)
- Periodic table – Wikipedia
- Periodic Table of Chemical Elements – American Chemical Society
- Periodic Table – Royal Society of Chemistry
- Periodic table | Definition, Elements, Groups, Charges, Trends, & Facts – Britannica
- Interactive Periodic Table of Elements – Fisher Scientific
- 3D Periodic Table – Experiments with Google
- The Photographic Periodic Table of the Elements – Photographic Periodic Table
- Periodic Table of Elements and Chemistry – Chemicool
- Periodic Table of Elements – Live Science
- How to Read the Periodic Table – American Museum of Natural History
- Interactive Periodic Table of Elements – Idaho National Laboratory (.gov)
- Interactive Periodic Table of the Elements, in Pictures and Words – Periodic Table of the Elements, in Pictures
- Periodic Table of the Elements – Minerals Education Coalition
- The Periodic Table – Introductory Chemistry – UEN Digital Press
- Clickable Periodic Table of the Elements – ThoughtCo
- Periodic table – chart of all chemical elements – Lenntech
- Periodic Table of Elements – Los Alamos National Laboratory (.gov)
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
