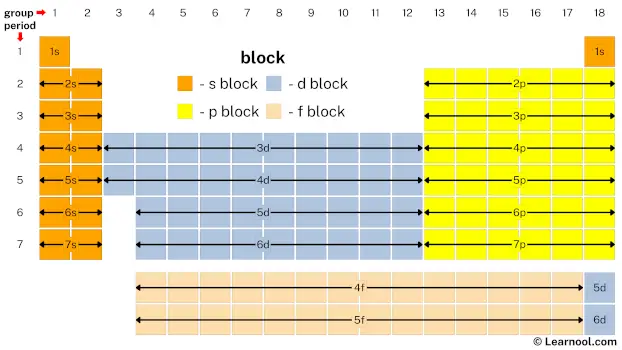
A block in the periodic table refers to a group of elements that exhibit similar chemical and physical properties based on their electron configurations. The arrangement of the periodic table is based on the increasing atomic number and electron configuration of the elements, with rows representing periods and columns representing groups. The periodic table is divided into four blocks, namely s-block, p-block, d-block, and f-block, which correspond to specific regions of the periodic table where electrons are filled in the s, p, d, and f subshells. The s-block elements have their outermost electrons in the s-subshell, while the p-block elements have their outermost electrons in the p-subshell, and so on.
The names of these blocks are based on the spectroscopic notation of an electron’s azimuthal quantum number, with sharp (s), principal (p), diffuse (d), and fundamental (f) representing values 0, 1, 2, and 3, respectively. The elements within each block share similar electron configurations and exhibit similar chemical and physical properties. However, the g-block, with its potential elements, has not yet been observed, although the letter “g” was chosen for its name as it follows the alphabetical order after f. The arrangement of blocks in the periodic table provides a framework for predicting the properties and behaviors of elements, as well as for identifying the trends in their properties, such as atomic radius, ionization energy, electronegativity, and chemical reactivity, across the periodic table.
The concept of blocks in the periodic table was first proposed by French spectroscopist Charles Janet in 1928. Janet’s work was based on the understanding that the order in which atomic orbitals are filled with electrons determines the chemical and physical properties of the element. He proposed the division of the periodic table into blocks to reflect this arrangement. Although the block concept was initially met with resistance from some quarters, it has since become an essential tool for understanding the trends in the properties and reactivity of elements across the periodic table. Janet’s contribution to the development of the periodic table continues to be recognized and celebrated in the scientific community.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – s block | – p block | ||
| – d block | – f block | ||
The periodic table is divided into four blocks: s, p, d, and f. The s-block contains 14 elements, the p-block contains 36 elements, the d-block contains 40 elements, and the f-block contains 28 elements.
s-block
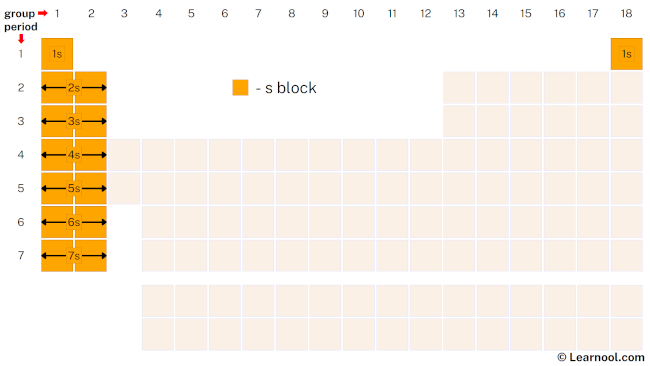
The s-block is a region of the periodic table containing elements from the first two groups (columns) of the periodic table, which are the alkali metals and alkaline earth metals, as well as the nonmetals hydrogen and helium. These elements have two s electrons in their outermost electron shell, which is the s-subshell. They are called the “s-block elements” because the outermost electrons are in s-orbitals.
The s-block elements are highly reactive and tend to form ionic compounds with nonmetals. The alkali metals in group 1 have a single outer s electron, making them the most reactive metals in the periodic table. They have low melting and boiling points and are soft enough to be easily cut with a knife. Alkaline earth metals in group 2 have two outer s electrons and are less reactive than the alkali metals. They have higher melting and boiling points and are harder than the alkali metals. The s-block elements are used in a variety of applications such as in batteries, construction materials, and the production of fertilizers. For instance, calcium is commonly used in the production of cement for construction, and lithium-ion batteries use lithium as a key component.
p-block
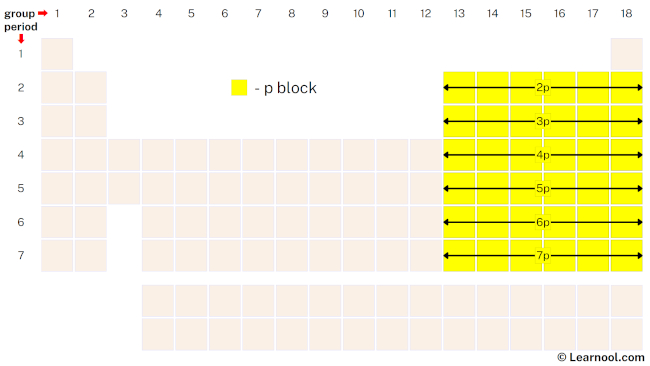
The p-block is the region of the periodic table that includes six columns, groups 13 to 18, and contains 36 elements, making it the second-largest block. Elements in the p-block have their outermost electrons in the p-subshell, which can hold a maximum of six electrons. The valence electrons in p-block elements participate in a wide range of chemical reactions, making these elements crucial to many aspects of chemistry and materials science. Helium is also in group 18 but is not considered part of the p-block, as it has only two electrons in its outermost s-subshell.
The p-block elements include some of the most important and abundant elements in nature, such as carbon, nitrogen, and oxygen. These elements form the backbone of many organic compounds, which are essential to life. In addition, the p-block includes elements with diverse properties, ranging from the noble gases, which are generally unreactive, to the highly reactive halogens and chalcogens. The p-block also includes some important metals, such as aluminum and tin, as well as nonmetals like sulfur and selenium. The properties of p-block elements are influenced by factors such as electronegativity, atomic size, and ionization energy, which change in predictable ways across the block.
In addition to the main-group elements in the p-block, there are also some elements known as the poor metals or metalloids included in the p-block. These elements (boron, silicon, germanium, arsenic, antimony, and tellurium) have some properties of metals and some properties of nonmetals, and their placement in the periodic table has been a topic of debate among chemists.
The p-block elements have a variety of uses in industry and technology. For example, carbon, the basis of organic chemistry, is a p-block element found in all living organisms. Nitrogen, another p-block element, is used in the production of fertilizers, while oxygen is essential for respiration and combustion. The halogens, which are also p-block elements, have a range of uses in disinfectants, bleaches, and other chemicals. Finally, the noble gases in the p-block have low reactivity and are used in applications such as lighting, welding, and as cryogens.
d-block
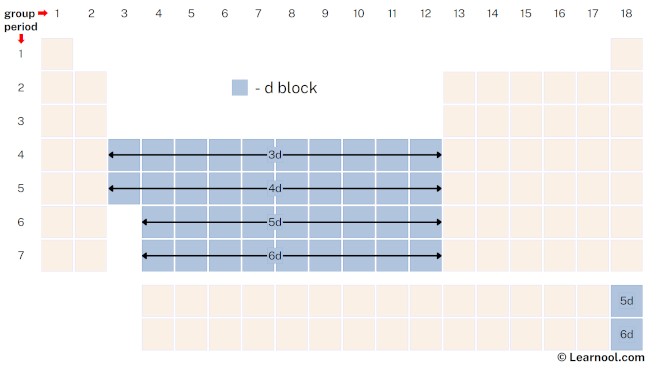
The d-block, also called the transition metals, is located in the center of the periodic table and includes 40 elements spanning 10 columns or groups, from group 3 to group 12. These elements have their outermost electrons in the d-subshell, which can hold up to 10 electrons. The d-block elements have many unique properties, including high melting and boiling points, multiple oxidation states, and the ability to form complex ions and compounds.
The transition metals in the d-block are used in a wide range of applications, from construction materials to electronics. Iron, the most common transition metal, is used in the production of steel, while copper is an important electrical conductor. Other d-block elements like titanium, vanadium, and chromium are used in alloys to improve properties like strength and corrosion resistance.
The d-block elements also play important roles in biological systems, with several transition metals being essential micronutrients. Iron is a key component of hemoglobin, which carries oxygen in the blood, while copper is involved in various enzyme reactions. The d-block elements have also been studied for their potential in catalyzing chemical reactions, particularly in industrial processes.
The unique properties of the d-block elements are largely due to their partially filled d-orbitals, which allow for a variety of bonding arrangements and complex coordination geometries. As a result, these elements have diverse chemistry and exhibit a wide range of physical and chemical properties. The knowledge of d-block elements’ behavior is crucial to many areas of chemistry and materials science.
f-block
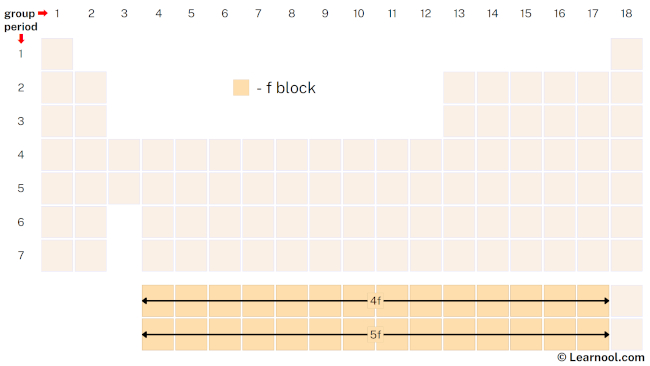
The f-block, also known as the inner transition metals, is a section of the periodic table that includes the lanthanide and actinide series of elements. These elements are located beneath the d-block and placed at the bottom of the periodic table, and they do not belong to any specific group. The lanthanide series consists of elements 57 to 71, while the actinide series includes elements 89 to 103. Lutetium and lawrencium, despite being lanthanides and actinides, are not included in the f-block because they have a filled f shell, which distinguishes them from the other elements in their respective series.
The f-block elements have unique electron configurations, with their valence electrons occupying the f-subshell. Because of the shielding effect of the inner electrons, the outermost electrons in f-block elements are relatively isolated from the rest of the atom, giving rise to some unusual properties. For example, many f-block elements have high melting and boiling points, as well as magnetic and electrical properties.
The lanthanides and actinides are often referred to as rare-earth elements, but this name can be misleading, as some of these elements are actually quite abundant. However, they are typically found in low concentrations in the Earth’s crust and can be difficult to extract and purify. The f-block elements have a wide range of uses, including in magnets, batteries, and nuclear reactors. Some f-block elements also have important applications in medicine and imaging, such as gadolinium-based contrast agents used in magnetic resonance imaging (MRI).
g-block
The g-block is a theoretical extension of the periodic table of elements beyond the f-block. It is predicted to occur after the superactinides, elements beyond the actinides in the periodic table. The g-block is also known as the “extended periodic table” or the “hypothetical g-block.”
There is currently no experimental evidence for the existence of elements in the g-block, and their properties are largely unknown. The g-block is of interest to theoretical chemists and physicists who study the electronic structure and chemical properties of hypothetical elements in this region of the periodic table. While the g-block is not a formally recognized region of the periodic table, it represents an area of active research and speculation in the field of theoretical chemistry.
Properties
The elements in the s-block have one or two valence electrons, which are held relatively close to the nucleus. As a result, s-block elements tend to have low ionization energies and electronegativities, making them good reducing agents. The alkali metals (group 1) are highly reactive and tend to form ionic compounds with nonmetals, while the alkaline earth metals (group 2) are less reactive and tend to form ionic compounds with oxygen and other chalcogens.
The p-block elements display diverse chemical properties due to the differing numbers of valence electrons they have. Group 14-18 consists of nonmetals that are generally brittle and have low melting and boiling points, while groups 13-16 comprise soft metals that also have low melting and boiling points. The metalloids in groups 13-17 have intermediate properties and can display characteristics of both metals and nonmetals, depending on the specific conditions.
Elements in the d-block are characterized by having valence electrons in the d-subshell, which can accommodate up to 10 electrons. This unique electron configuration results in d-block elements often having high melting and boiling points, high densities, and strong thermal and electrical conductivity. The transition metals found in groups 3-12 of the periodic table are typically known for being hard, dense, and strong, and they often form brightly colored compounds due to the presence of partially filled d-orbitals.
The f-block elements have valence electrons in the f-subshell, which can hold up to 14 electrons. Due to their unique electronic configuration, these elements have a wide range of properties, with many displaying notable magnetic and optical behavior. The lanthanides are generally soft, silvery metals that are often used as catalysts in various chemical reactions, while the actinides are predominantly radioactive and have numerous nuclear and chemical applications.
Related
More topics
- Block (periodic table)
- Group (periodic table)
- Period (periodic table)
- Metal
- Alkali metal
- Alkaline earth metal
- Lanthanide
- Actinide
- Transition metal
- Post-transition metal
- Metalloid
- Nonmetal
- Reactive nonmetal
- Noble gas
External links
- Block (periodic table) – Wikipedia
- Periodic Table Blocks of Elements – Science Notes and Projects
- Blocks in periodic table Study Guide – Inspirit VR
- What Are Element Blocks on the Periodic Table? – ThoughtCo
- 6.8: Blocks of the Periodic Table – Chemistry LibreTexts
- Blocks of the Periodic Table – CK-12
- S-Block Elements in the Periodic Table – Study.com
- Block on the Periodic Table? – elements – Chemistry Stack Exchange
- The F-Block: An introduction – Scienceline
- What determines the blocks in a periodic table? – Quora
- Modern Periodic Table | s,p,d,f Blocks Elements – Pinterest
- s, p, d and f Blocks of the Periodic Table Chemistry Tutorial – AUS-e-TUTE
- 41.THE PERIODIC TABLE – s,p,d,f blocks – Madoverchemistry
- Building the Periodic Table Block by Block – HowStuffWorks
- p-Block Elements – Chemistry – Brightstorm
- The P-block: The Right Side of the Periodic Table – Wondrium Daily
- p-Block Elements – Chemistry Learner
- Periodic Table of the Elements – Blocks – Gordon England
- Evolution and understanding of the d-block elements in the periodic table – RSC Publishing
- Which block of the periodic table is the largest? – Socratic
- About: Block (periodic table) – DBpedia
- Block of the elements – Photographic Periodic Table
- P-Block Elements – Bartleby
- Question Video: Identifying the Area of the Periodic Table That Corresponds to the s Block – Nagwa Limited
- Blocks of the Periodic Table | Chemistry for Non-Majors – Course Hero
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
