
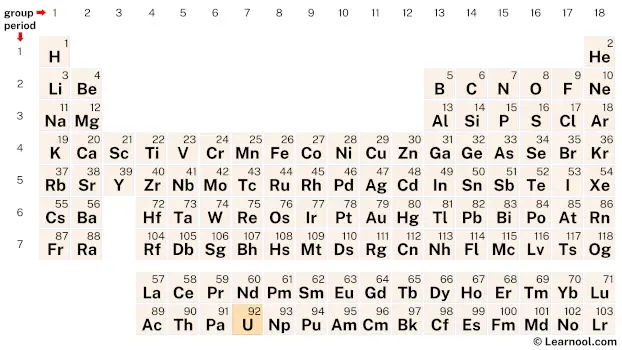
Uranium (U) is a chemical element of the periodic table, located in the period 7, and has the atomic number 92. It is the fourth element in the actinide series. It is a silvery-white metal which is named after the planet Uranus. It is counted as one of the radioactive elements.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – f block |
Uranium (U) is located on the periodic table in the actinide series, which is a group of elements located at the bottom of the table. Specifically, in period 7, between protactinium (Pa) and neptunium (Np).
Element information
 |
|
 |
|
| Origin of name | named after planet Uranus |
| Symbol | U |
| Atomic number (Z) | 92 |
| Atomic mass | 238.02891 u |
| Block | f-block |
| Period | 7 |
| Classification | Actinide |
| Atomic radius | 156 pm |
| Covalent radius | 196±7 pm |
| Melting point | 1132.2 ℃, 2070 ℉, 1405.3 K |
| Boiling point | 4131 ℃, 7468 ℉, 4404 K |
| Electron configuration | [Rn] 5f3 6d1 7s2 |
| Electrons per shell | 2, 8, 18, 32, 21, 9, 2 |
| Crystal structure | Orthorhombic |
| Phase at r.t | Solid |
| Density near r.t | 19.1 g/cm3 |
| Natural occurrence | Primordial |
| Oxidation state | +4, +6 |
| Electronegativity (Pauling scale) | 1.38 |
| Protons Neutrons Electrons |
92 146 92 |
| Learn how to find: Uranium protons neutrons electrons | |
| CAS number | 7440-61-1 |
| Discovered by | Martin Heinrich Klaproth in 1789 |
History

The history of uranium dates back to the late 18th century when German chemist Martin Heinrich Klaproth identified an unknown element from pitchblende ore and named it “uranium” after the planet Uranus. In 1841, French chemist Eugene-Melchior Peligot isolated the metal for the first time by reducing uranium tetrachloride with potassium. The discovery of radioactivity in the late 19th century led to the realization that uranium was a radioactive element, and it became widely used in nuclear power and weapons.
During the 20th century, uranium was extensively mined and processed for military and civilian applications. In the 1930s, nuclear fission was discovered, and the potential for nuclear energy was realized. This led to a significant increase in the demand for uranium, and the development of nuclear reactors and weapons programs in several countries. The Manhattan Project, a research project that produced the first nuclear weapons during World War Ⅱ, was one of the most significant events in the history of uranium.
Today, uranium is primarily used for nuclear power generation, accounting for about 10% of the world’s electricity production. It is also used in some medical procedures and as a component in certain industrial processes. However, concerns over the safety and environmental impact of nuclear power have led to increased interest in renewable energy sources in recent years.
Occurrence and production
Uranium is a naturally occurring element found in the earth’s crust, typically at concentrations of 2-4 parts per million. It is more abundant than silver and about as common as tin. Uranium can be found in many different minerals, including uraninite, carnotite, autunite, and coffinite, as well as in phosphate rock, lignite, and monazite sands.
The primary method for mining uranium is through underground or open-pit mining techniques, depending on the location and geology of the deposit. Uranium ore is typically mined as an oxide, commonly called yellowcake, and then processed to extract the uranium.
The production of uranium involves several steps, including mining, milling, conversion, and enrichment. After the uranium is mined, it is crushed and ground into a fine powder. The uranium is then treated with chemicals to extract the uranium from the rock, resulting in yellowcake. The yellowcake is then processed further to produce uranium hexafluoride gas, which is used in the enrichment process. The enrichment process involves increasing the concentration of uranium-235, the isotope used in nuclear reactors, by separating it from the more abundant uranium-238 isotope. This can be done using gas diffusion, gas centrifugation, or laser enrichment technologies. Once enriched, the uranium is formed into fuel rods and used in nuclear power plants.
Properties
Physical properties
Uranium is a silvery-white metal that is highly reactive and easily oxidizes in air.
It has a melting point of 1132.2 ℃ and a boiling point of 4131 ℃.
Uranium has a density of 19.1 g/cm3, making it one of the densest naturally occurring elements.
Chemical properties
Uranium is a highly reactive metal that readily combines with other elements to form compounds.
It is a relatively soft metal and can be easily cut with a knife.
Uranium is highly soluble in acids and reacts with water to form uranium oxide and hydrogen gas.
It has a wide range of oxidation states, ranging from -3 to +6, with +4 and +6 being the most common.
Toxicity
Uranium is highly toxic, primarily due to its radioactivity.
It can cause both chemical toxicity and radiological toxicity, with the latter being the most dangerous.
Exposure to uranium can lead to a variety of health problems, including kidney damage, lung cancer, and genetic mutations.
The toxicity of uranium depends on various factors such as its chemical form, the route of exposure, and the duration and intensity of exposure.
Isotopes
Uranium has 27 known isotopes, including three naturally occurring isotopes: uranium-238, uranium-235, and uranium-234.
Uranium-235 is the only naturally occurring fissile isotope and is used in nuclear reactors and weapons.
Nuclear properties
Uranium is a radioactive element and undergoes alpha, beta, and gamma decay.
Its most stable isotope, uranium-238, has a half-life of about 4.5 billion years.
Uranium can also undergo nuclear fission, releasing a significant amount of energy.
Applications
Uranium is primarily used as fuel in nuclear reactors to generate electricity. The process involves the nuclear fission of uranium atoms, which releases large amounts of energy.
Uranium is also used in the production of nuclear weapons, as it is a key material for nuclear fission bombs and thermonuclear weapons.
Uranium isotopes are used in medicine for various diagnostic and therapeutic purposes, such as radiation therapy for cancer treatment and imaging techniques for examining organs and tissues.
Uranium isotopes are used in the geological dating of rocks and minerals, as the decay of uranium into lead can provide information on the age of the material.

Uranium oxide is used as a colorant in glass and ceramics, producing a yellow or green hue depending on the concentration used.
Uranium is used as an alloying agent in the production of high-strength and high-temperature alloys for use in aircraft and other high-performance applications.
Uranium is used as a radiation source for Geiger counters and other radiation detection devices.
Interesting facts
Uranium is the heaviest naturally occurring element on earth and has the highest atomic number (92) of all naturally occurring elements.
Uranium is a silvery-white metal that is ductile, malleable, and paramagnetic. It is also radioactive and has a very long half-life, making it useful in many scientific applications.
Uranium was discovered in 1789 by German chemist Martin Heinrich Klaproth, who named it after the planet Uranus.
Uranium is used as a fuel for nuclear reactors and in the production of nuclear weapons. It is also used in the production of isotopes for medical and industrial purposes.
The element has several isotopes, some of which are highly unstable and decay into other elements. Uranium-238, which makes up 99.3% of all natural uranium, has a half-life of about 4.5 billion years.
Uranium was used in the production of the first atomic bomb, which was detonated in the New Mexico desert in 1945. Since then, the element has played a significant role in world events and global politics.
Uranium is also found in trace amounts in many rocks, soils, and waters, and is used by scientists to determine the age of rocks and fossils through radiometric dating.
Uranium is also the only naturally occurring element that has fissile isotopes, meaning they can sustain a nuclear chain reaction.
Uranium has been used in traditional medicine in some cultures, particularly in India and China, where it is believed to have therapeutic properties. However, the use of uranium for medicinal purposes is controversial and not scientifically proven.
Related
More elements
External links
- https://www.rsc.org/periodic-table/element/92/uranium
- https://www.britannica.com/science/uranium
- https://en.wikipedia.org/wiki/Uranium
- https://pubchem.ncbi.nlm.nih.gov/element/Uranium
- https://www.ducksters.com/science/chemistry/uranium.php
- https://www.livescience.com/39773-facts-about-uranium.html
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
