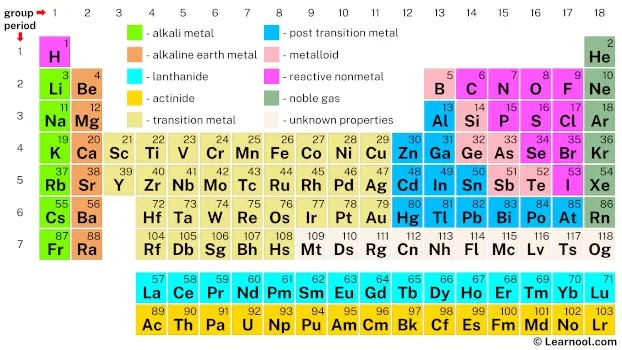
In chemistry, a group (also referred to as a family) is a column of elements in the periodic table. Each group contains elements that share the same number of valence electrons and exhibit similar chemical and physical properties. The periodic table is divided into 18 numbered groups, each having a unique set of elements. Groups can also be identified by the topmost element in the group, or by a specific name.
The 18 groups in the periodic table are numbered from 1 to 18 according to the modern numbering system recommended by the International Union of Pure and Applied Chemistry (IUPAC) since 1988. However, there are different systems of group numbering which can cause confusion as the same number may be assigned to different groups depending on the system being used. Nevertheless, the modern numbering system is generally accepted by the chemistry community.
Groups 1 to 2 are known as the s-block, groups 3 to 12 are known as the d-block, groups 13 to 18 are known as the p-block, and the 14 f-block columns are located between groups 2 and 3, but are not numbered. Each block contains elements that have the same number of valence electrons in their outermost shells. The properties of elements within a group vary with their atomic number, and the properties of elements in the same group are more similar to each other than to those in other groups.
In addition to being identified by number, each group may also have a specific name. For example, group 1 is commonly known as the alkali metals, while group 17 is known as the halogens. These names are used to describe the common properties and behavior of elements in that particular group. It is important to note that not all groups have a specific name, and some elements may belong to multiple groups depending on the criteria used for classification.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – alkali metal | – post-transition metal | ||
| – alkaline earth metal | – metalloid | ||
| – lanthanide | – reactive nonmetal | ||
| – actinide | – noble gas | ||
| – transition metal | – unknown properties | ||
The periodic table consists of 18 numbered groups, organized from left to right. The main groups include metals (which consist of alkali metals, alkaline earth metals, lanthanides, actinides, transition metals, and post-transition metals), metalloids, and nonmetals (which include reactive nonmetals and noble gases).
However, the f-block elements between groups 2 and 3 (lanthanides and actinides) do not have any group number. These elements are commonly referred to as the inner transition metals and are placed below the main table to conserve space.
In addition, the elements with atomic numbers 109 to 118 are referred to as unknown chemical properties, because they are highly unstable and can only be produced artificially in a lab.
Group names
| Group 1 | ⅠA | ⅠA | lithium group | hydrogen and alkali metals | |
| Group 2 | ⅡA | ⅡA | beryllium group | alkaline earth metals | |
| Group 3 | ⅢA | ⅢB | scandium group | ||
| Group 4 | ⅣA | ⅣB | titanium group | ||
| Group 5 | ⅤA | ⅤB | vanadium group | ||
| Group 6 | ⅥA | ⅥB | chromium group | ||
| Group 7 | ⅦA | ⅦB | manganese group | ||
| Group 8 | Ⅷ | ⅧB | iron group | ||
| Group 9 | Ⅷ | ⅧB | cobalt group | ||
| Group 10 | Ⅷ | ⅧB | nickel group | ||
| Group 11 | ⅠB | ⅠB | copper group | coinage metals | |
| Group 12 | ⅡB | ⅡB | zinc group | volatile metals | |
| Group 13 | ⅢB | ⅢA | boron group | triels (from the Greek word “tri”, three, Ⅲ) | icosagens |
| Group 14 | ⅣB | ⅣA | carbon group | tetrals (from the Greek word “tetra”, four, Ⅳ) | crystallogens adamantogens merylides |
| Group 15 | ⅤB | ⅤA | nitrogen group | pnictogens pentels (from the Greek word “penta”, five, Ⅴ) |
|
| Group 16 | ⅥB | ⅥA | oxygen group | chalcogens | |
| Group 17 | ⅦB | ⅦA | fluorine group | halogens | |
| Group 18 | 0 | ⅧA | helium group or neon group | noble gases | aerogens |
Properties and trends
The properties of elements in the periodic table are fundamental to the study of chemistry. Each group in the periodic table shares similar chemical and physical properties, making it a useful tool for predicting the behavior of elements. For example, the alkali metals in group 1, including lithium, sodium, and potassium, all have one valence electron, which makes them highly reactive and good conductors of heat and electricity.
The physical and chemical properties of elements in each group are unique and often have practical applications. For example, the alkaline earth metals in group 2, including magnesium and calcium, are important components in the manufacturing of construction materials and batteries. The noble gases in group 18, including helium and neon, are used in lighting and as insulators due to their low reactivity.
Trends in the periodic table, such as atomic radius and electronegativity, are important for understanding the behavior of elements in each group. For example, as you move from left to right across a period, the atomic radius decreases due to increasing nuclear charge. Similarly, as you move from the bottom to the top of a group, the electronegativity increases, which affects the reactivity of the elements.
The trends in the periodic table can be further explained with the help of examples. For instance, the halogens in group 17, including chlorine and iodine, become less reactive as you move from top to bottom. This is because the atomic radius increases, making it harder for them to attract electrons and form bonds. Another example is the transition metals in group 3 to group 12, including iron and copper, which become less reactive as you move from left to right. This is because the electronegativity of the elements increases, making it harder for them to lose electrons and form bonds.
Naming and numbering
The naming and numbering of groups in the periodic table has been a subject of discussion and has undergone evolution over time. There are three different systems of group numbering that have been used in the past. However, the modern numbering system has been recommended by the International Union of Pure and Applied Chemistry (IUPAC) since 1988, and it is widely used today. In this system, groups are numbered from 1 to 18, with the f-block elements (lanthanides and actinides) not assigned any number.
Before the modern system, there were two different systems of naming and numbering of groups. The first system was developed by the Chemical Abstract Service (CAS) and was more popular in the United States. This system assigned group numbers from ⅠA to ⅧA for the main-group elements and from ⅠB to ⅧB for the transition elements. The second system was developed by IUPAC and was more popular in Europe. This system used Roman numerals with the letters A and B to distinguish between the left (A) and right (B) side of the periodic table.
The modern system of group numbering is now widely accepted by the chemistry community, although there is still some debate over the placement of elements 1 and 2 (hydrogen and helium) and the inner transition metals. Despite this, the modern system has several advantages, including being more systematic and easier to use than the previous systems.
In addition to the numbered groups, some groups in the periodic table are also identified by their topmost element or a specific name. For example, group 16 is commonly referred to as the “oxygen group” or “chalcogens,” while group 18 is called the “noble gases.” Another example is the “iron group,” which usually refers to group 8 but can also include iron, cobalt, and nickel or some other set of elements with similar chemical properties.
Related
More topics
- Block (periodic table)
- Group (periodic table)
- Period (periodic table)
- Metal
- Alkali metal
- Alkaline earth metal
- Lanthanide
- Actinide
- Transition metal
- Post-transition metal
- Metalloid
- Nonmetal
- Reactive nonmetal
- Noble gas
External links
- Group (periodic table) – Wikipedia
- Groups of the periodic table (video) – Khan Academy
- Group | Definition & Facts – Britannica
- How the Periodic Table groups the elements – Live Science
- 2.5 The Periodic Table – Chemistry – IU Pressbooks
- Periodic Table: Periods, Groups, and Families – Chemistry Learner
- 2.5: The Periodic Table – Chemistry LibreTexts
- Periodic Table – University of Queensland
- How to Read the Periodic Table | Groups & Periods – ChemTalk
- Properties of Periodic Table of Element Groups – ThoughtCo
- Reading the Periodic Table – California State University, Northridge
- The Structure and Meaning of the Periodic Table: Groups – Breaking Atom
- Group – LANL Periodic Table – Los Alamos National Laboratory (.gov)
- Groups in the Periodic Table | Secondaire – Alloprof
- Groups of the periodic table – Mammoth Memory
- Periodic Table of the Elements – Periodic Table Groups – Cool Periodic Table
- Periodic Table Groups and Periods – Science Notes and Projects
- Group (periodic table) Facts for Kids – Kids encyclopedia facts
- Interactives . The Periodic Table . Groups – Annenberg Learner
- Periodic table, main group elements – New World Encyclopedia
- Periodic table Groups Explained !! (With 1-18 Group Names) – Periodic Table Guide
- Groups of Elements – CK-12 Foundation
- Recalling the Meaning of a Group in the Periodic Table – Nagwa Limited
- What are the names of the 8 groups in the periodic table? – Quora
- What are periodic table groups and what do they represent? – Socratic
- Periodic Table Groups – Pediabay
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
