Noble gases, also known as inert gases, are a group of elements in the periodic table that are known for their low reactivity. The six noble gases are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). These elements are called noble gases because of their tendency to not react with other elements or compounds, and their name “inert gas” reflects this property as well.
Oganesson (Og) is a relatively new element discovered in 2002 and is currently the heaviest element in the periodic table. Its placement in the table is still debated, with some sources considering it a noble gas due to some of its chemical properties resembling those of noble gases. However, its electron configuration is predicted based on theoretical calculations, and is not experimentally confirmed, leading to some uncertainty in its classification. Nonetheless, its unique properties make it an intriguing subject for further study.
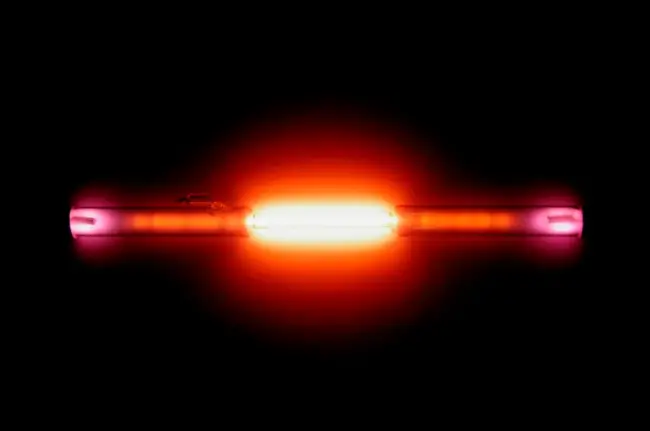
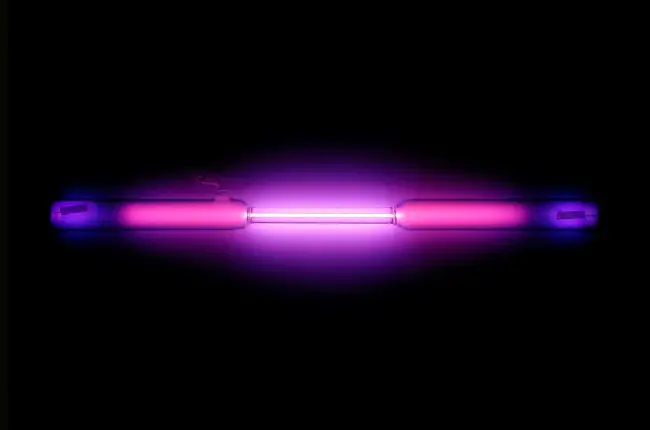
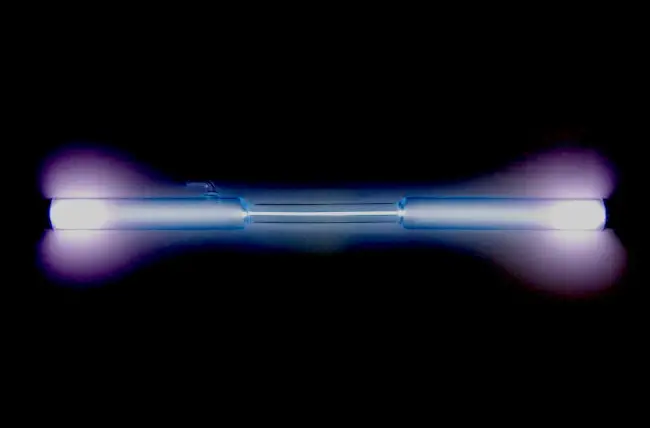
Noble gases have some unique properties that make them stand out from other elements on the periodic table. They are all odorless, colorless, and tasteless, and have low boiling and melting points. They are also non-flammable and non-reactive under normal conditions, making them useful in applications where the risk of explosion or corrosion is high. Another interesting property of noble gases is their ability to emit light when an electric current is passed through them, which is why they are often used in lighting applications.
The noble gases have a variety of important applications in industry and technology. Helium, for example, is used in cryogenics, as well as in nuclear magnetic resonance imaging (MRI) machines. Argon is used in welding and metal fabrication, while xenon is used in lighting and anesthesia. Radon, on the other hand, is a naturally occurring radioactive gas and is a significant health hazard when it accumulates in enclosed spaces.
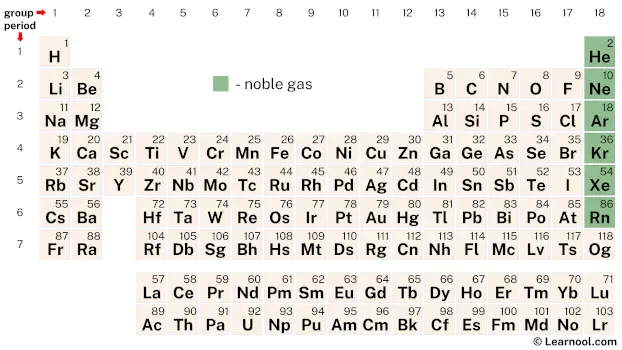
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – noble gas |
Noble gases are a group of six chemical elements – helium, neon, argon, krypton, xenon, and radon – located in group 18 of the periodic table.
History

Noble gases have a rich history, with multiple scientists contributing to the discovery of these elements. The term “Edelgas,” which means “noble gas” in German, was first coined by the chemist Hugo Erdmann in 1898. However, it was the British chemist William Ramsay and his colleague Lord Rayleigh who first identified the noble gases as a distinct group of elements in the late 19th century. Ramsay is credited with discovering four of the six noble gases: neon, argon, krypton, and xenon.
Helium was first discovered in 1868 by the French astronomer Jules Janssen during a solar eclipse. However, it was the British astronomer Norman Lockyer who first suggested that the spectral line observed during the eclipse was due to a new element, which he named helium. Neon was discovered in 1898 by Ramsay and Morris Travers when they were studying liquefied air. They noticed an unexpected orange-red glow when an electrical discharge was passed through the gas, leading them to identify it as a new element.
Argon, the third noble gas discovered, was identified by Ramsay and Rayleigh in 1894. They found that a sample of nitrogen extracted from the air was denser than expected, indicating the presence of another gas. They named the new gas “argon” from the Greek word for “lazy” because of its inertness. Krypton, the fourth noble gas, was discovered by the British scientists William Ramsay and Morris Travers in 1898. They were investigating the residue left after evaporating nearly all components of liquid air, and found a new component with a very bright yellow-green spectral emission line that they named krypton.
Xenon, the fifth noble gas, was discovered in 1898 by Ramsay and Travers. They were analyzing the remaining components of liquid air and found a new gas with a blue spectral emission line. The name “xenon” was derived from the Greek word for “stranger” or “foreigner” to reflect its rarity and unusual properties. Friedrich Ernst Dorn, a German physicist, discovered radon, the sixth and final noble gas, in 1900 while studying the radioactive decay of radium. He observed that radium produced a gas, which he named “radium emanation,” that further decayed into another gas, which was later identified as radon.
Occurrence and production
Noble gases are present in trace amounts in the Earth’s atmosphere and are obtained from the air using various methods. Helium, the second lightest element, is the most abundant noble gas in the universe but is relatively rare on Earth. It is extracted from natural gas wells, where it is formed by the radioactive decay of uranium and thorium. In some cases, it can also be extracted from the air using a process called cryogenic distillation, which involves cooling air to very low temperatures and separating the different gases based on their boiling points. Neon is the fifth most abundant element in the universe and is obtained from the air by liquefaction and fractional distillation. Argon, the third most abundant gas in the Earth’s atmosphere, is also obtained from the air by the process of fractional distillation.
Krypton and xenon, which are present in even smaller amounts in the atmosphere than argon, are obtained from air by cryogenic distillation. These gases are separated from each other and from the other gases in air by taking advantage of the small differences in their boiling points at very low temperatures. Radon, on the other hand, is a radioactive gas that is formed by the decay of uranium and thorium in the Earth’s crust. It is present in rocks, soil, and water and can seep into buildings, where it can accumulate and pose a health risk.
Properties
Physical properties
Noble gases are colorless, odorless, tasteless, and nonflammable gases at room temperature.
They have very low boiling and melting points, and exist as monatomic gases under normal conditions.
Noble gases have low densities and are poor conductors of heat and electricity.
They have the lowest electronegativity of all elements, and have high ionization energies, which means they require a lot of energy to lose an electron and become a cation.
Chemical properties
Noble gases are inert and have very low reactivity due to their stable electron configuration.
They do not readily form compounds with other elements, with the exception of krypton, xenon and radon which can form a few stable compounds under certain conditions.
Optical properties
Noble gases are used in various lighting applications, such as neon signs and fluorescent lamps, due to their ability to produce bright and vibrant colors.
They are also used in excimer lasers which produce intense, short pulses of light for industrial and medical applications.
Toxicity
Noble gases are generally considered to be non-toxic and non-reactive, making them safe to handle and use in various applications.
However, some noble gases such as radon can be radioactive and harmful if they are present in high concentrations.
Applications
Lighting
Noble gases such as neon, helium, and argon are used in various lighting applications, including neon signs, fluorescent lamps, and incandescent bulbs.
Welding
Argon is widely used in welding applications because it provides an inert atmosphere, which prevents the oxidation of the metal being welded.
Medical imaging
Helium is used in medical imaging applications, such as magnetic resonance imaging (MRI), because it is non-toxic and does not react with the body’s tissues.
Cooling
Helium is used as a coolant in various applications, including nuclear reactors, MRI machines, and space vehicles, because it has a very low boiling point and is non-reactive.
Diving
Helium and other noble gases are used in diving applications, including deep-sea diving and diving in contaminated water, because they do not react with the body and do not cause narcosis at high pressures.
Laser technology
Noble gases are used in various types of lasers, including excimer lasers, which are used in industrial and medical applications.
Plasma technology
Noble gases are used in plasma display panels (PDPs) and plasma TVs, which use a combination of noble gases and other elements to produce bright, high-quality images.
Leak detection
Helium is often used as a tracer gas for leak detection in various industrial applications, including pipelines, refrigeration systems, and air conditioning systems.
Space exploration
In space applications, noble gases are commonly used for their unique properties. Helium, for example, serves as a coolant in space vehicles, while xenon is used in ion thrusters to provide propulsion.
Interesting facts
Helium is the only noble gas that doesn’t solidify at standard pressure and temperature conditions.
Neon is used in some high-voltage indicators and advertising signs because it glows bright orange-red when an electrical current passes through it.
Radon is the densest noble gas and is the only radioactive one, making it a health hazard in high concentrations.
Argon is the third-most abundant gas in the Earth’s atmosphere, after nitrogen and oxygen.
Most noble gases, except krypton and xenon, do not have electronegativity values.
The first noble gas to be discovered was helium in 1868 by French astronomer Jules Janssen and British astronomer Joseph Norman Lockyer.
The name “noble gases” comes from the fact that they are very unreactive, similar to the behavior of nobility in society.
Noble gases are often used in lighting applications, such as in neon lights and fluorescent lamps.
The noble gases are the only group of elements that exists as gases under standard conditions.
Related
More topics
- Block (periodic table)
- Group (periodic table)
- Period (periodic table)
- Metal
- Alkali metal
- Alkaline earth metal
- Lanthanide
- Actinide
- Transition metal
- Post-transition metal
- Metalloid
- Nonmetal
- Reactive nonmetal
- Noble gas
Also read
External links
- Noble gas – Wikipedia
- Noble gas | Definition, Elements, Properties, Characteristics, & Facts – Britannica
- Noble Gases – Periodic Table – ChemTalk
- Noble Gas – Nuclear Regulatory Commission (.gov)
- Group 18: Properties of Nobel Gases – Chemistry LibreTexts
- Group 8A — The Noble or Inert Gases – Angelo State University
- Elements & Periodic Table: Noble Gases – Chem4Kids
- Noble Gases – Chemical Elements.com
- Noble Gases Properties, Uses and Sources – ThoughtCo
- THE PERIODIC TABLE – THE NOBLE GASES – ACS Publications
- Noble Gas Atom – an overview – ScienceDirect
- History of noble gases | Feature – Chemistry World
- Noble Gases – Springer
- Noble Gases – CK-12
- Noble Gases – University of Illinois Urbana-Champaign
- Noble Gases – Concept – Chemistry Video – Brightstorm
- Noble gas Definition & Meaning – Merriam-Webster
- Noble Gases Their Physical And Chemical Characteristics – Jack Westin
- Noble Gases – Diamond Light Source
- Halogens and Noble Gases – Periodic Table – Turito
- Interactives . The Periodic Table . Groups – Annenberg Learner
- Group 18 The Noble Gases – Kent’s Chemistry
- Noble gas Definition & Meaning – Dictionary.com
- What Are Noble Gases? Definition and Properties – Science Notes and Projects
- Chemistry for Kids: Elements – The Noble Gases – Ducksters
- Noble Gases – Encyclopedia.com
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.