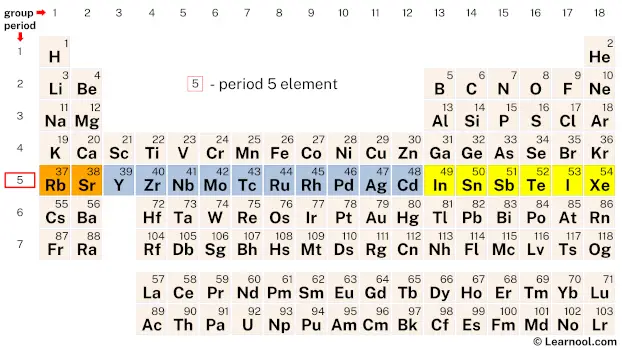
Period 5 elements are a group of chemical elements found in the fifth row or period of the periodic table. This period consists of 18 elements, starting from rubidium (Rb) with atomic number 37 and ending with xenon (Xe) with atomic number 54. The period 5 elements display a wide range of physical and chemical properties that make them essential in a variety of applications. These elements have unique electronic configurations that influence their reactivity, making them useful in industries such as electronics, energy, and medicine.
Period 5 elements, like those in periods 3 and 4, can also have expanded octets. However, not all period 5 elements follow the octet rule. Transition metals, for example, do not conform to the octet rule. Similarly, some p-block elements in period 5, such as Sn, do not require 8 electrons in their valence shell. Only elements beyond Sn, including Sb, Te, I, and Xe, have the ability to form an expanded octet.
The physical and chemical properties of period 5 elements can vary widely; as a result, they are useful in a range of applications. For example, some period 5 elements, such as niobium and molybdenum, have high melting points and are often used in the production of superalloys for jet engines and gas turbines. Other elements, such as iodine and xenon, have applications in medicine and lighting technology. These diverse applications highlight the importance of understanding the physical and chemical properties of period 5 elements and their versatile role in various industries.
Period 5 elements, such as zirconium and ruthenium, have shown potential applications in the field of catalysis according to recent studies. Zirconium-based catalysts have been found to be effective in the production of polymers and plastics, while ruthenium-based catalysts have shown promise in the synthesis of various organic compounds. As research in the field of catalysis continues to advance, the unique properties of period 5 elements are expected to play an increasingly important role in the development of new and more efficient catalytic processes.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| 5 | – period 5 element |
Period 5 on the periodic table consists of 18 elements, beginning with the alkali metal Rubidium and ending with the noble gas Xenon.
| Rubidium (Rb) | |
| Strontium (Sr) | |
| Yttrium (Yt) | |
| Zirconium (Zr) | |
| Niobium (Nb) | |
| Molybdenum (Mo) | |
| Technetium (Tc) | |
| Ruthenium (Ru) | |
| Rhodium (Rh) | |
| Palladium (Pd) | |
| Silver (Ag) | |
| Cadmium (Cd) | |
| Indium (In) | |
| Tin (Sn) | |
| Antimony (Sb) | |
| Tellurium (Te) | |
| Iodine (I) | |
| Xenon (Xe) | |
Element information
s-block
Rubidium

Rubidium is a soft, silvery-white metal that belongs to the alkali metal group. It has an atomic number of 37 and is the first element in period 5. Rubidium is highly reactive, and it rapidly oxidizes in air. It has a low melting point and can easily form alloys with other metals. Rubidium has a variety of uses, including in atomic clocks and as a getter in vacuum tubes. It is also used in some medical applications, such as in positron emission tomography (PET) scans.
Strontium

Strontium is a soft, silver-white metal that belongs to the alkaline earth metal group. It has an atomic number of 38 and is the second element in period 5. Strontium has similar properties to calcium, and it can replace calcium in biological processes. It is used in the production of ferrite magnets and as a component in flares and fireworks, where it produces a deep red color. Strontium-90, a radioactive isotope of strontium, has been used in cancer treatments to destroy cancerous cells. Strontium is also sometimes added to toothpaste to help strengthen teeth and prevent cavities.
d-block
Yttrium

Yttrium is a silvery-white metal that has a metallic luster. It is a rare-earth metal that is relatively stable in air but reacts with water and acids. Yttrium is often found in minerals such as yttria, monazite, and xenotime. It has a high melting point and is a good conductor of electricity and heat, which makes it useful in various applications such as electronics and nuclear reactors. Yttrium also has medical applications, including as a tracer in positron emission tomography (PET) scans. Its unique properties and abundance in the Earth’s crust make yttrium an important element for both industrial and scientific purposes.
Zirconium

Zirconium (Zr) is a grayish-white metal with an atomic number of 40. It is a strong and ductile metal with a high melting point and excellent corrosion resistance. Zirconium is often used in nuclear reactors due to its ability to withstand high temperatures and radiation. It is also used in the production of ceramic materials, such as zirconia, which is used in dental and medical implants. Zirconium has other applications as well, including as a component in alloys for aerospace and defense industries, and in the production of fireworks and flares due to its bright white light emission.
Niobium

Niobium is a silver-gray, soft, and ductile metal with a high melting point. It is highly resistant to corrosion and oxidation at high temperatures, making it useful in a variety of industrial applications. Niobium is commonly used in the production of superalloys for jet engines, gas turbines, and rocket engines, as well as in the production of high-strength steel alloys.
Molybdenum

Molybdenum is a silvery-white, hard, and ductile metal with a high melting point. It has excellent strength and corrosion resistance at high temperatures, making it a valuable material in a wide range of applications. Molybdenum is used in the production of alloys, including high-strength steels and superalloys for use in the aerospace and defense industries. It is also used in the production of electrical contacts, filaments, and electrodes, as well as in the chemical and petroleum industries.
Technetium

Technetium is a silvery-gray metal that is highly radioactive, and it is the lightest element that has no stable isotopes. It was the first artificially produced element and was discovered in 1937 by a group of scientists led by Italian physicist Emilio Segrè. Technetium has a variety of uses, including as a tracer in medical imaging to diagnose diseases and to measure blood flow. It is also used as a corrosion inhibitor in steel alloys and as a catalyst in chemical reactions.
Ruthenium

Ruthenium is a rare and precious metal that is often found with other platinum group metals. It has a silvery-white appearance and is highly resistant to corrosion and oxidation. Ruthenium has a number of industrial applications, including as a hardening agent in alloys used for electrical contacts, wear-resistant coatings, and electrical capacitors. It is also used as a catalyst in production of chemicals and pharmaceuticals. In addition, ruthenium has potential applications in cancer treatment due to its ability to bind to cancer cells and trigger their death.
Rhodium

Rhodium is a rare, silvery-white transition metal with atomic number 45. It is a hard and durable metal that has a high melting point, which makes it useful in high-temperature applications such as furnace windings and aircraft parts. Rhodium is also used as a catalyst in various chemical reactions, such as the production of acetic acid and hydrogenation of organic compounds. It is a valuable metal and is often used in jewelry and decorative items due to its brilliant white color and resistance to tarnishing.
Palladium

Palladium is another silvery-white transition metal with atomic number 46. It has similar physical and chemical properties to other elements in the platinum group, such as platinum and rhodium. Palladium is most commonly used as a catalyst in catalytic converters for automobiles, where it helps to reduce harmful emissions. It is also used in the production of electronics, dental fillings, and jewelry due to its excellent resistance to corrosion and tarnishing. In recent years, palladium has become a valuable investment metal due to its increasing demand and limited supply.
Silver

Silver is a lustrous, white metal with the highest electrical conductivity of any element. It is highly reflective and has a high thermal conductivity, making it useful in a variety of applications, including electrical contacts, mirrors, and silverware. Silver also has antimicrobial properties, which make it useful in medical applications such as wound dressings and catheters. In addition, silver compounds have been used in photography and as a catalyst in chemical reactions.
Cadmium

Cadmium is a soft, bluish-white metal with low melting and boiling points. It is used primarily as a coating for other metals to prevent corrosion. Cadmium is also used in rechargeable batteries, pigments, and in the production of plastic products. However, cadmium is toxic and can accumulate in the environment, which poses a threat to human health. As a result, its use in many products has been restricted or banned in some countries.
p-block
Indium

Indium is a soft, silvery-white metal that is relatively rare in the Earth’s crust. It has a melting point of 156.59 ℃ and a boiling point of 2072 ℃. Indium is used in a variety of applications due to its unique properties, including its ability to conduct electricity and resist corrosion. It is commonly used in the production of touch screens, LCD displays, and solar panels. Indium tin oxide is a widely used material in the fabrication of transparent conductive coatings for these applications. Indium is also used as a dopant in semiconductor materials, such as gallium arsenide, to enhance their electrical properties.
Tin

Tin is a soft, silvery-white metal that has been known and used by humans for thousands of years. It has a melting point of 231.93 ℃ and a boiling point of 2602 ℃. Tin is used in many alloys, including bronze, pewter, and solders, due to its low melting point and ability to form strong bonds with other metals. It is also used as a protective coating on other metals to prevent corrosion. Tin is used in the production of food packaging materials, such as tin cans, as it is non-toxic and does not react with food or beverages. In addition, tin is used in the production of certain types of glass, such as float glass, to improve their properties.
Antimony

Antimony is a metalloid, meaning that it has properties of both metals and nonmetals. It has a silvery-white color and is brittle, with a crystalline texture. Antimony is a poor conductor of heat and electricity. It is commonly used as a flame retardant and as an alloying agent in lead-acid batteries. Antimony is also used in the production of semiconductors, infrared detectors, and ammunition.
Tellurium

Tellurium is a brittle, silvery-white metalloid that has a metallic luster. It is a rare element and is often found in combination with other elements, such as gold and copper. Among the chalcogens, which are the oxygen-family elements, tellurium has the highest melting and boiling points, and it is a poor conductor of heat and electricity. It has applications in the production of solar panels, thermoelectric generators, and rewritable optical disks. Tellurium is also used in the metallurgy of iron and stainless steel to improve their machinability.
Iodine

Iodine is a nonmetal. It is a dark gray, shiny solid at room temperature, but it easily sublimates to a violet-colored gas when heated. Iodine is an essential element for human health and is used in the production of thyroid hormones, which regulate metabolism. It is also used as an antiseptic, and in the production of certain pharmaceuticals, dyes, and photographic chemicals. Iodine has a wide range of applications, including water treatment, food fortification, and nuclear power generation.
Xenon
Xenon is a rare, colorless, and odorless gas. It is one of the noble gases and has seven stable isotopes. Xenon is one of the heaviest noble gas and is considered to be one of the least reactive chemical elements. It is used in lighting, particularly in high-intensity discharge lamps, and as a general anesthetic. In nuclear reactors, it can be used as a coolant and a neutron absorber. Xenon has also been used in spacecraft propulsion systems due to its ability to be ionized and accelerated by electric and magnetic fields.
Properties
Melting and boiling point
The melting and boiling points of period 5 elements can vary widely due to differences in their atomic and molecular structures. The transition metals of this period, such as niobium and molybdenum, have high melting points due to strong metallic bonding. The nonmetals of this period, such as iodine and xenon, have low melting points due to weak van der Waals forces.
Electronegativity
Period 5 elements have varying electronegativities, with some elements such as iodine being highly electronegative, while others such as niobium and molybdenum have relatively low electronegativities. Electronegativity generally increases as we move across the period from left to right, with iodine being the most electronegative element in the period.
Oxidation state
Period 5 elements can exhibit a wide range of oxidation states, from -3 to +7. This variability is due to the availability of five valence electrons, which can participate in a range of chemical reactions. The elements in the middle of the period, such as niobium and molybdenum, have the most varied oxidation states.
Density
The density of period 5 elements increases as we move across the period due to the increasing size and atomic weight of the elements. The elements in the middle of the period, such as niobium and molybdenum, have relatively high densities, while elements on the left and right sides of the period, such as rubidium and xenon, have lower densities.
Reactivity
The reactivity of period 5 elements varies widely depending on the element. Across the period, the transition metals such as niobium and molybdenum tend to have higher reactivity than the nonmetals like iodine and tellurium. This is due to the presence of unpaired electrons in the transition metals. Left to right, the reactivity generally decreases, with the elements on the left of the period being more reactive than those on the right.
Related
More topics
- Period 1 element
- Period 2 element
- Period 3 element
- Period 4 element
- Period 5 element
- Period 6 element
- Period 7 element
External links
- Period 5 element – Wikipedia
- About: Period 5 element – DBpedia
- Period 5 Element – Academic Accelerator
- Period 5 element – Elements Wiki | Fandom
- Period 5 element – wikidoc
- Period 5 element – Wikiwand
- Period 5 element – Academic Kids
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
