
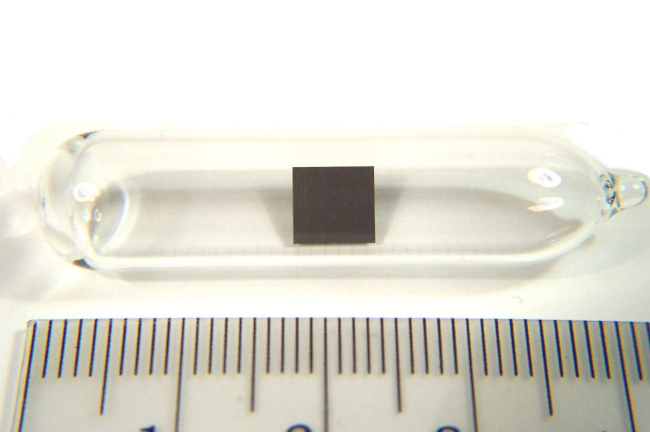
Thorium (Th) is a chemical element of the periodic table, located in the period 7, and has the atomic number 90. It is the second element in the actinide series. It is a silvery metal that turns gray or black, when it is exposed to air. It is named after Thor, the Norse god of thunder. It is counted as one of the radioactive elements.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – f block |
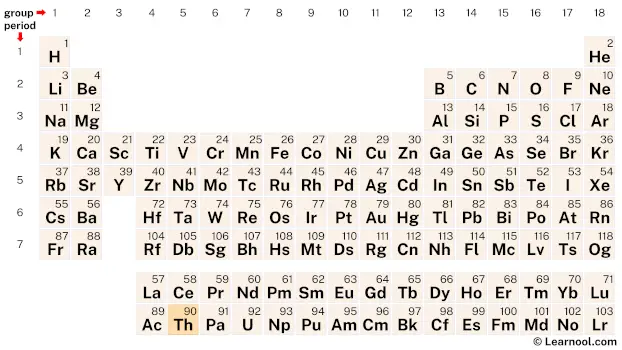
Thorium is a member of the actinide series, a group of elements located at the bottom of the periodic table. It can be found in period 7, between actinium (Ac) and protactinium (Pa).
Element information
 |
|
 |
|
| Origin of name | named after Thor, the Norse god of thunder |
| Symbol | Th |
| Atomic number (Z) | 90 |
| Atomic mass | 232.03806 u |
| Block | f-block |
| Period | 7 |
| Classification | Actinide |
| Atomic radius | 179.8 pm |
| Covalent radius | 206±6 pm |
| Melting point | 1750 ℃, 3182 ℉, 2023 K |
| Boiling point | 4788 ℃, 8650 ℉, 5061 K |
| Electron configuration | [Rn] 6d2 7s2 |
| Electrons per shell | 2, 8, 18, 32, 18, 10, 2 |
| Crystal structure | Face-centered cubic (fcc) |
| Phase at r.t | Solid |
| Density near r.t | 11.7 g/cm3 |
| Natural occurrence | Primordial |
| Oxidation state | +1 |
| Electronegativity (Pauling scale) | 1.3 |
| Protons Neutrons Electrons |
90 142 90 |
| Learn how to find: Thorium protons neutrons electrons | |
| CAS number | 7440-29-1 |
| Discovered by | Jöns Jacob Berzelius in 1828 |
History

Thorium was discovered in 1828 by the Swedish chemist Jöns Jacob Berzelius, who named it after Thor, the Norse god of thunder. However, it was not until the late 19th century that its properties were fully understood.
In 1898, Marie Curie and her husband Pierre discovered that thorium, along with uranium, was a source of radioactivity. This led to the discovery of other radioactive elements and the field of nuclear chemistry. Thorium was also found to be a potent source of nuclear energy and was used in early nuclear research and experimentation.
During the early 20th century, thorium was primarily used in gas mantles for lighting, as it produced a bright and long-lasting light when heated. It was also used in alloys with other metals, such as magnesium and aluminum, to increase their strength and resistance to corrosion. However, its use in these applications has declined in recent years due to safety concerns and the development of more efficient alternatives.
Today, thorium is primarily used in the nuclear energy industry as a fuel for nuclear reactors. It has the potential to produce more energy than uranium and is much more abundant in the Earth’s crust, making it an attractive alternative to traditional nuclear fuels. Research into thorium-based nuclear reactors is ongoing, with many scientists and engineers considering it a promising avenue for sustainable and clean energy production.
Occurrence and production
Thorium is a naturally occurring element that can be found in the Earth’s crust at a concentration of approximately 7.2 parts per million. It is typically found in minerals such as monazite and thorite, as well as in some other rare earth minerals. The largest reserves of thorium are found in countries such as the United States, Australia, and India.
In terms of production, thorium is typically obtained as a byproduct of mining and refining rare earth metals. It can also be extracted from monazite sands using various chemical processes. The production of thorium is currently limited, as its use in nuclear reactors is still in the research and development stage.
Additionally, thorium can be produced by the irradiation of natural thorium in a nuclear reactor, resulting in the formation of the fissile isotope uranium-233. However, this process is not currently economically feasible on a large scale.
Properties
Physical properties
Thorium is a silver-gray, soft, and ductile metal. It has a density of 11.7 grams per cubic centimeter, melting point of 1750 degrees Celsius, and boiling point of 4788 degrees Celsius.
Chemical properties
Thorium is a reactive metal, which slowly reacts with oxygen, water, and most acids. It is resistant to alkalis and does not dissolve in hydrochloric acid or nitric acid. It readily forms alloys with iron, nickel, and other metals.
Radioactivity
Thorium is a radioactive element, and its most stable isotope, thorium-232, undergoes alpha decay to produce radium-228. The decay chain of thorium-232 produces a number of other radioactive isotopes, including radon-220 and polonium-216, which are significant sources of radiation exposure.
Isotopes
Thorium has 32 known isotopes, with atomic masses ranging from 207 to 238. Thorium-232 is the most stable isotope, with a half-life of 14.05 billion years.
Oxidation states
Thorium has a characteristic +4 oxidation state, which is the most stable in aqueous solutions. It can also exhibit oxidation states of +3, +2, and +1 under certain conditions.
Applications
Nuclear energy
Thorium can be used as a fuel in nuclear reactors, particularly in molten salt reactors (MSRs). MSRs have several advantages over traditional nuclear reactors, such as improved safety, reduced nuclear waste, and a higher conversion efficiency.
Alloying agent
Thorium can be added to alloys to improve their strength, ductility, and resistance to corrosion. Thorium-containing alloys are used in the aerospace and automotive industries, as well as in the manufacture of heat-resistant equipment.
Gas mantles
Thorium oxide was once used in the manufacture of gas mantles for lamps. The thorium oxide was impregnated into fabric or other materials, which were then burned, leaving behind a white ash that glowed brightly.
Medical applications
Thorium has been used in some medical applications, particularly in radiation therapy. However, due to its radioactivity and potential health risks, its use in medicine is limited.
Catalysts
Thorium compounds can act as catalysts in certain chemical reactions, particularly those involving the conversion of hydrocarbons.
Interesting facts
Thorium was discovered in 1828 by Swedish chemist Jöns Jakob Berzelius, who named it after the Scandinavian god of thunder, Thor.
Thorium is a naturally occurring radioactive element that can be found in small amounts in soil, rocks, and minerals such as monazite and thorite.
Thorium is an alpha-emitting material, which means it emits positively charged particles when it decays. These particles can be stopped by a sheet of paper or skin, but they can be dangerous if inhaled or ingested.
Thorium can be used as a nuclear fuel in nuclear reactors, although it is not currently widely used for this purpose. One of the advantages of using thorium as a fuel is that it produces less long-lived radioactive waste than other nuclear fuels.
Thorium is lighter than uranium, and it is not fissile on its own. However, it can be used as a fertile material in a breeder reactor to produce fissile material such as uranium-233.
Thorium has been suggested as a potential alternative to uranium for nuclear power generation. It is more abundant than uranium and produces less radioactive waste, although there are still technological and economic barriers to its widespread use.
Thorium has luminescent properties and is used in gas lantern mantles, where it is heated to incandescence to produce light.
Thorium can also be used for geological dating, as it has a long half-life and is present in many minerals. By measuring the amount of thorium present in a mineral, scientists can determine the age of the rock or mineral.
Related
More elements
External links
- https://www.rsc.org/periodic-table/element/90/thorium
- https://en.wikipedia.org/wiki/Thorium
- https://www.britannica.com/science/thorium
- https://www.livescience.com/39686-facts-about-thorium.html
- https://www.chemicool.com/elements/thorium.html
- https://pubchem.ncbi.nlm.nih.gov/element/Thorium
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
