
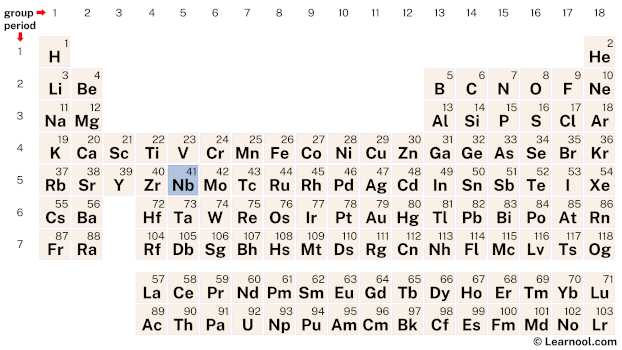
Niobium (Nb) is a chemical element of the periodic table, located in the group 5 and the period 5, and is having the atomic number 41. It is a ductile, silvery-white transition metal, which is named after Niobe, the daughter of king Tantalus in Greek mythology.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – d block |
Niobium is a d-block element, situated in the fifth column of the periodic table, between zirconium (Zr) and molybdenum (Mo). It has the atomic number 41 and is denoted by the symbol Nb.
Element information
 |
|
 |
|
| Origin of name | named after Niobe, the daughter of king Tantalus in Greek mythology |
| Symbol | Nb |
| Atomic number (Z) | 41 |
| Atomic mass | 92.90638 u |
| Block | d-block |
| Group | 5 |
| Period | 5 |
| Classification | Transition metal |
| Atomic radius | 146 pm |
| Covalent radius | 164±6 pm |
| Melting point | 2477 ℃, 4491 ℉, 2750 K |
| Boiling point | 4744 ℃, 8571 ℉, 5017 K |
| Electron configuration | [Kr] 4d4 5s1 |
| Electrons per shell | 2, 8, 18, 12, 1 |
| Crystal structure | Body-centered cubic (bcc) |
| Phase at r.t | Solid |
| Density near r.t | 8.57 g/cm3 |
| Main isotopes | Niobium-93 |
| Natural occurrence | Primordial |
| Oxidation state | +5 |
| Electronegativity (Pauling scale) | 1.6 |
| Protons Neutrons Electrons |
41 52 41 |
| Learn how to find: Niobium protons neutrons electrons | |
| CAS number | 7440-03-1 |
| Discovered by | Charles Hatchett in 1801 |
History

Niobium was first discovered in 1801 by the English chemist Charles Hatchett. He initially found an unknown mineral in a sample of a brownstone from Connecticut and named it columbium. However, Hatchett’s analysis was inconclusive, and the mineral was later determined to be the same as tantalum, another element with similar properties.
It wasn’t until 1844 that Heinrich Rose, a German chemist, identified niobium in a sample of tantalite from Sweden. He named the element niobium after the mythical Greek figure Niobe, the daughter of Tantalus, in order to avoid confusion with tantalum.
Niobium remained a relatively obscure element until the 20th century, when its properties were better understood and it became more widely used in industry.
In 1903, the American chemist Charles DuBois patented the process for producing niobium metal by reduction of its oxide with aluminum. During World War Ⅱ, the demand for niobium increased as it was used to make high-strength, low-alloy steels for military applications.
Occurrence and production
Niobium is a relatively abundant element in the Earth’s crust, with an estimated concentration of 20 parts per million (ppm). It is usually found in the minerals pyrochlore and columbite, which are widely distributed around the world. The largest deposits of niobium are located in Brazil, where the country accounts for more than 90% of the world’s niobium production. Other significant producers of niobium include Canada, Australia, and Nigeria.
The production of niobium typically involves several stages, including mining, concentration, and refining. In the first stage, the ore is mined and crushed to a manageable size. The next step involves separating the niobium-containing minerals from the rest of the ore. This is done through a combination of gravity separation, magnetic separation, and flotation. The resulting concentrate is then processed through a series of chemical reactions to produce pure niobium metal.
Properties
Niobium is a soft, gray, ductile metal with a high melting point.
It has a high melting point, making it suitable for high-temperature applications such as jet engines and gas turbines.
Niobium is a good conductor of electricity and is paramagnetic at room temperature.
It has a relatively low thermal neutron capture cross-section.
Niobium is resistant to corrosion and oxidation, due to the formation of a protective oxide layer on the surface.
It has a density of 8.57 g/cm3 and a boiling point of 4744 ℃.
Niobium has a strong affinity for oxygen and is commonly found in minerals such as columbite and pyrochlore.
Applications
High-performance alloys
Niobium is commonly used as an alloying element with other metals such as iron, nickel, and titanium to produce alloys with high strength, corrosion resistance, and other desirable properties.
Superconducting magnets and wires
Niobium-tin and niobium-titanium alloys are used to create superconducting magnets and wires, which are used in a variety of scientific and medical applications, such as particle accelerators and MRI machines.
Steelmaking
Small amounts of niobium can be added to steel to increase its strength and toughness, particularly in high-stress applications such as pipelines, oil rigs, and bridges.
Electronics
Niobium oxide is used in the manufacture of capacitors for electronic devices such as smartphones and laptops, as it has a high dielectric constant and is able to store large amounts of energy in a small space.
Jet engine components
Niobium alloys are used to create high-temperature-resistant components for jet engines, such as turbine blades and combustion chambers.
Jewelry
Niobium is a popular material for making jewelry, particularly body jewelry such as earrings and body piercings, as it is hypoallergenic and does not cause skin irritation or allergic reactions.
Interesting facts
Niobium was named after Niobe, a figure from Greek mythology who was the daughter of King Tantalus and wife of Amphion.
The symbol for niobium is Nb, which comes from its former name, “columbium” (Cb). It was only in the 1940s that the name was officially changed to niobium.
Niobium is one of the five major refractory metals (along with tungsten, molybdenum, tantalum, and rhenium) that have a melting point above 2000 ℃.
Niobium is a superconductor at very low temperatures, and is used in various applications such as MRI machines, particle accelerators, and nuclear reactors.
Niobium is also used in the aerospace industry, where its high melting point and resistance to corrosion make it an ideal material for jet engines and other components.
Brazil is the largest producer of niobium, accounting for more than 90% of the world’s production. Other significant producers include Canada, Australia, and Nigeria.
Niobium has a low neutron cross-section, which makes it useful in nuclear reactors as a cladding material for fuel rods.
Niobium is also used in the production of high-strength steel alloys, as well as in various other applications such as jewelry, electronics, and welding electrodes.
Related
More elements
External links
- https://www.rsc.org/periodic-table/element/41/niobium
- https://en.wikipedia.org/wiki/Niobium
- https://www.britannica.com/science/niobium
- https://www.livescience.com/34682-niobium.html
- https://www.chemicool.com/elements/niobium.html
- https://study.com/learn/lesson/niobium-element-properties-uses-facts.html
- https://pubchem.ncbi.nlm.nih.gov/element/Niobium
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
