
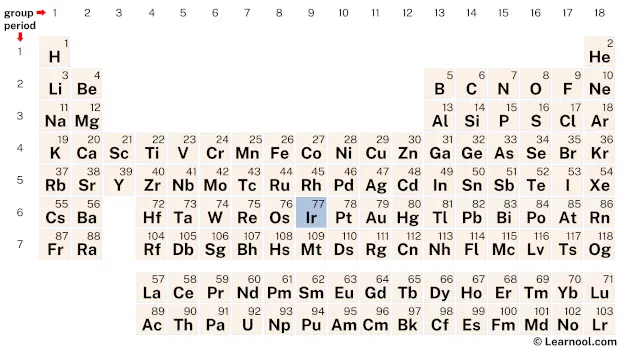
Iridium (Ir) is a chemical element of the periodic table, located in the group 9 and the period 6, and is having the atomic number 77. It is a hard, brittle, silvery-white transition metal, which is named after Iris, Greek goddess of the rainbow.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – d block |
Iridium is a d-block element, situated in the ninth column of the periodic table, between osmium (Os) and platinum (Pt). It has the atomic number 77 and is denoted by the symbol Ir.
Element information
 |
|
 |
|
| Origin of name | named after Iris, Greek goddess of rainbow |
| Symbol | Ir |
| Atomic number (Z) | 77 |
| Atomic mass | 192.217 u |
| Block | d-block |
| Group | 9 |
| Period | 6 |
| Classification | Transition metal |
| Atomic radius | 136 pm |
| Covalent radius | 141±6 pm |
| Melting point | 2446 ℃, 4435 ℉, 2719 K |
| Boiling point | 4130 ℃, 7466 ℉, 4403 K |
| Electron configuration | [Xe] 4f14 5d7 6s2 |
| Learn how to write: Iridium electron configuration | |
| Electrons per shell | 2, 8, 18, 32, 15, 2 |
| Learn how to draw: Iridium Bohr model | |
| Crystal structure | Face-centered cubic (fcc) |
| Phase at r.t | Solid |
| Density near r.t | 22.56 g/cm3 |
| Main isotopes | Iridium-191, Iridium-193 |
| Natural occurrence | Primordial |
| Oxidation state | +3, +4 |
| Electronegativity (Pauling scale) | 2.20 |
| Protons Neutrons Electrons |
77 115 77 |
| CAS number | 7439-88-5 |
| Discovered by | Smithson Tennant in 1803 |
History

The history of iridium dates back to the early 19th century, when it was first discovered in 1803 by the English chemist Smithson Tennant.
Tennant discovered iridium while analyzing the residues left behind after dissolving platinum ore in a mixture of nitric and hydrochloric acids. He found that the remaining black powder contained two new elements, which he named iridium and osmium. Tennant’s discovery was significant because it expanded the list of known elements and helped establish the field of analytical chemistry.
Despite its discovery in 1803, it took almost 50 years for iridium to be isolated in its metallic form. In 1842, the French chemist Henri Sainte-Claire Deville developed a method to extract iridium from its oxide form by heating it with a mixture of potassium chloride and sodium fluoride. This process is still used today to extract iridium from its ores.
Since its discovery, iridium has found various applications in modern technology due to its unique properties. Its most famous use is in the iridium layer found in the boundary between the Cretaceous and Paleogene geological periods, which provides evidence for the impact theory of the extinction of the dinosaurs. Iridium is also used in electronics, jewelry, and as a catalyst in chemical reactions.
Occurrence and production
Iridium is one of the rarest elements on Earth, occurring at an average concentration of only 0.001 ppm in the Earth’s crust. It is primarily obtained from platinum ores and is found in association with other platinum-group metals. The largest reserves of iridium are located in South Africa, Russia, and Canada.
Iridium is obtained as a by-product of nickel and copper mining and refining. It is extracted from the metal’s ores through a series of chemical reactions, which involves precipitation, fusion, and separation. The process is complex and time-consuming, which makes iridium one of the most expensive metals in the world.
Properties
Iridium is a dense, hard, brittle, and lustrous transition metal.
It has a high melting point of 2446 ℃ and a boiling point of 4130 ℃, making it one of the most heat-resistant elements.
Iridium has a very high density of 22.56 g/cm3, making it the second densest element after osmium.
It is highly corrosion-resistant and is not attacked by most acids, including aqua regia, which is a mixture of hydrochloric acid and nitric acid that can dissolve gold and platinum.
Iridium is a member of the platinum group metals (PGMs) and shares many of the same properties as platinum, such as its high melting point and resistance to corrosion.
It has a face-centered cubic crystal structure and is not magnetic.
Iridium is a good electrical conductor and is used in electrical contacts and spark plugs.
It also has a very high modulus of elasticity, which makes it useful in the manufacture of hard, wear-resistant alloys used in the production of pen tips, compass bearings, and other applications where durability is important.
Applications
Iridium is mainly used in high-temperature applications due to its exceptional resistance to heat and corrosion.
It is used in the production of crucibles and other equipment used in high-temperature experiments and analysis.
Iridium is also used in the production of electrical contacts and spark plugs due to its high melting point and resistance to wear and corrosion.
It is used as a catalyst in chemical reactions, especially in the production of fertilizers and in the refining of crude oil.
Iridium is used in the production of jewelry due to its hardness, durability, and resistance to tarnish.
It is often alloyed with platinum to produce high-quality, durable jewelry pieces.
Iridium-192, a radioactive isotope of iridium, is used in radiography to detect defects in metal parts and structures.
Interesting facts
Iridium is one of the rarest elements in the Earth’s crust, with an abundance of only 0.001 ppm.
It is often found with platinum and other platinum group metals in alluvial deposits in South Africa and in nickel-copper deposits in Canada and Russia.
Iridium has the highest melting point of all the platinum group metals, with a melting point of 2446 ℃.
One of the most well-known applications of iridium is its use in the production of high-performance spark plugs for aviation and racing engines.
Iridium is also used in the production of hard disks and other high-tech electronics due to its corrosion resistance and high melting point.
The iridium layer found in the K-Pg boundary, also known as the Cretaceous-Paleogene boundary, suggests that an asteroid impact caused the mass extinction of dinosaurs and many other species approximately 66 million years ago.
Related
More elements
External links
- https://www.rsc.org/periodic-table/element/77/iridium
- https://en.wikipedia.org/wiki/Iridium
- https://www.britannica.com/science/iridium3
- https://www.chemicool.com/elements/iridium.html
- https://pubchem.ncbi.nlm.nih.gov/element/Iridium
- https://education.jlab.org/itselemental/ele077.html
- https://study.com/learn/lesson/iridium-element-background-use.html
- https://www.livescience.com/39143-iridium.html
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
