
Californium (Cf) is a chemical element of the periodic table, located in the period 7, and has the atomic number 98. It is the tenth element in the actinide series. It is a soft, malleable, silvery-white metal which is named after the state of California, where it was first made. It is the sixth transuranium element and is counted as one of the radioactive elements.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
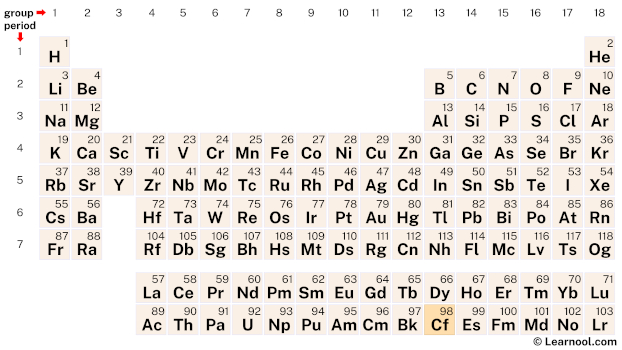
98 Cf Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – f block |
Californium (Cf) is located on the periodic table in the actinide series, which is a group of elements located at the bottom of the table. Specifically, in period 7, between berkelium (Bk) and einsteinium (Es).
Element information
 |
|
 |
|
| Origin of name | named after California |
| Symbol | Cf |
| Atomic number (Z) | 98 |
| Atomic mass | (251) |
| Block | f-block |
| Period | 7 |
| Classification | Actinide |
| Melting point | 900 ℃, 1652 ℉, 1173 K |
| Boiling point | 1470 ℃, 2678 ℉, 1470 K |
| Electron configuration | [Rn] 5f10 7s2 |
| Learn how to write: Californium electron configuration | |
| Electrons per shell | 2, 8, 18, 32, 28, 8, 2 |
| Learn how to draw: Californium Bohr model | |
| Crystal structure | Double hexagonal close-packed (dhcp) |
| Phase at r.t | Solid |
| Density near r.t | 15.1 g/cm3 |
| Natural occurrence | Synthetic |
| Oxidation state | +3 |
| Electronegativity (Pauling scale) | 1.3 |
| Protons Neutrons Electrons |
98 153 98 |
| Learn how to find: Californium protons neutrons electrons | |
| CAS number | 7440-71-3 |
| Discovered at | Lawrence Berkeley National Laboratory in 1950 |
History
Californium was first synthesized in 1950 by a team of researchers led by Stanley G. Thompson, Kenneth Street Jr., and Albert Ghiorso at the University of California, Berkeley. They bombarded curium-242 with alpha particles in a cyclotron, producing californium-245. The discovery was kept secret until 1952 due to its potential military applications.
The element was named after the state of California, where it was discovered, and is represented by the symbol Cf. Californium was the sixth transuranium element to be discovered and the first element to be produced in amounts large enough to see with the naked eye.
Since its discovery, californium has been extensively studied for its nuclear properties and potential applications. It has been used in various applications, including as a neutron source for scientific research and as a component in nuclear reactors.
Occurrence and production
Californium is not found naturally on Earth, as it is a synthetic element that can only be produced in a laboratory or nuclear reactor. However, trace amounts of californium have been detected in the fallout from nuclear weapons tests and in the spent fuel of nuclear reactors.
Californium can be produced by bombarding plutonium-239 or curium-244 with neutrons in a nuclear reactor. It can also be produced by bombarding americium-241 with alpha particles in a cyclotron. Californium is produced in very small quantities, and its isolation and purification are difficult and expensive processes. As a result, it has limited practical applications and is mainly used for scientific research.
Properties
Californium is a silvery-white metal that is highly radioactive and can emit alpha, beta, and gamma rays.
It has a melting point of 900 ℃ and a boiling point of approximately 1470 ℃.
Californium has an atomic number of 98 and a standard atomic weight of 251 g/mol.
It is a rare earth element and belongs to the actinide series.
Californium is paramagnetic, meaning it is weakly attracted to magnetic fields.
It has a relatively short half-life, with its most stable isotope, californium-251, having a half-life of about 900 years.
The element is highly reactive and can form compounds with a variety of other elements, including oxygen, nitrogen, and carbon.
Californium is one of the few elements that can undergo nuclear fission, and it has been used in experiments to study nuclear reactions and the production of heavy elements.
Due to its radioactivity and short half-life, Californium has no commercial applications outside of scientific research.
The element is extremely toxic and poses a significant health hazard, so its handling and storage require specialized equipment and procedures.
Applications
Californium-252 is a strong neutron emitter and can be used as a neutron source for various purposes, including in oil exploration, nuclear reactors, and medical applications.
Californium-252 is also used in cancer radiation therapy to treat tumors. The neutrons emitted by californium-252 can penetrate deep into the body and kill cancer cells.
Californium-252 can be used in nuclear reactors to start the fission process by emitting neutrons that cause other atoms to split, releasing more neutrons and energy.
Californium-252 can be used in nuclear forensics to identify nuclear materials and detect nuclear explosions.
Californium-252 is used in industrial radiography to detect defects in welds, castings, and other materials.
Californium-252 can be used in metal detectors to detect hidden metals by emitting neutrons that cause the metals to emit gamma rays.
Interesting facts
Californium is one of the most expensive chemical elements, costing about $27 million per gram.
It was named after the state of California, where it was first synthesized.
Californium-252 is a potent neutron emitter and is used in nuclear reactors, cancer treatment, and oil exploration.
Californium is the heaviest element that can be produced in nuclear reactors.
The half-life of Californium-252 is only 2.6 years, and it decays into lighter elements by emitting alpha particles.
Californium-252 is also used in industrial radiography and as a neutron source in moisture gauges and metal detectors.
It is a highly radioactive element and requires special handling and storage precautions.
Californium-252 is also used in the detection of gold and silver ores by the activation analysis method.
The University of California Radiation Laboratory played a significant role in the discovery and synthesis of Californium in the 1950s.
Californium has no known biological role and is highly toxic due to its radioactivity.
Related
More elements
External links
- https://www.rsc.org/periodic-table/element/98/californium
- https://en.wikipedia.org/wiki/Californium
- https://www.britannica.com/science/californium
- https://www.chemicool.com/elements/californium.html
- https://www.livescience.com/40272-facts-about-californium.html
- https://pubchem.ncbi.nlm.nih.gov/element/Californium
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
