
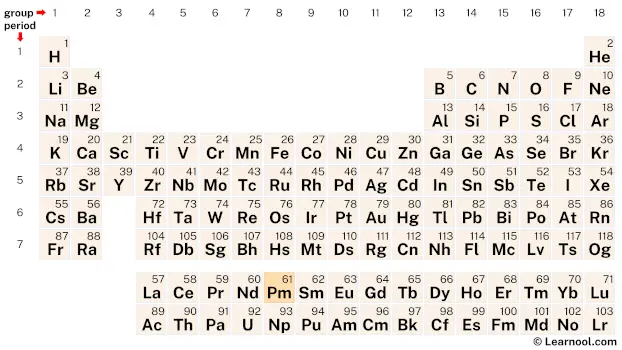
Promethium (Pm) is a chemical element of the periodic table, located in the period 6, and has the atomic number 61. It is the fifth element in the lanthanide series. It is a silvery-white metal that glows in the dark with a pale blue or green light. It is named after Prometheus, a Titan god of fire, in Greek mythology, who stole fire from the Gods and gave it to humans. It is a rare earth element that is not available freely in nature and is counted as one of the radioactive elements.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – f block |
Promethium is a member of the lanthanide series, a group of elements located at the bottom of the periodic table. It can be found in period 6, between neodymium (Nd) and samarium (Sm).
Element information
 |
|
| Origin of name | named after Prometheus, a Titan god of fire |
| Symbol | Pm |
| Atomic number (Z) | 61 |
| Atomic mass | (145) |
| Block | f-block |
| Period | 6 |
| Classification | Lanthanide |
| Atomic radius | 183 pm |
| Covalent radius | 199 pm |
| Melting point | 1042 ℃, 1908 ℉, 1315 K |
| Boiling point | 3000 ℃, 5432 ℉, 3273 K |
| Electron configuration | [Xe] 4f5 6s2 |
| Electrons per shell | 2, 8, 18, 23, 8, 2 |
| Learn how to draw: Promethium Bohr model | |
| Crystal structure | Double hexagonal close-packed (dhcp) |
| Phase at r.t | Solid |
| Density near r.t | 7.26 g/cm3 |
| Natural occurrence | From decay |
| Oxidation state | +3 |
| Electronegativity (Pauling scale) | 1.13 |
| Protons Neutrons Electrons |
61 84 61 |
| CAS number | 7440-12-2 |
| Discovered by | Charles D. Coryell, Jacob A. Marinsky, and Lawrence E. Glendenin in 1945 |
History
Promethium was predicted by the American chemist Charles D. Coryell and his colleagues in 1942, who believed that there must be an element between neodymium and samarium in the periodic table. They called this element “prometheum” (with the “e” later being changed to an “i” to match the name of the Titan from Greek mythology).
However, it was not until 1945 that the element was first discovered by Jacob A. Marinsky, Lawrence E. Glendenin, and Charles D. Coryell at Oak Ridge National Laboratory in Tennessee, USA. They isolated the element from nuclear fission products, and its existence was confirmed by its characteristic gamma radiation.
Promethium is the only element whose discovery was primarily due to the use of nuclear technology. Its discovery played a significant role in the development of nuclear science and technology.
Occurrence and production
Promethium is a rare earth element that is not found naturally on Earth. It is produced through nuclear reactions in nuclear reactors and through particle accelerators.
The most common method of producing promethium is through the irradiation of neodymium-142 with neutrons to produce neodymium-143, which then decays into promethium-143.
Another method involves the irradiation of other rare earth elements, such as samarium or europium, to produce promethium-147. Once produced, promethium can be extracted from nuclear reactor fuel rods through ion exchange chromatography or solvent extraction techniques.
The production of promethium is limited, as it is only produced in very small quantities in nuclear reactors, and it has very few practical applications.
Properties
Promethium is a radioactive element that has no stable isotopes and is only found in trace amounts in nature.
Its physical and chemical properties are not well known due to its rarity and radioactivity.
It has a silvery-white metallic appearance and is malleable and ductile.
Promethium has an atomic number of 61 and belongs to the lanthanide series of elements.
Its electron configuration is [Xe] 4f5 6s2, with the 4f shell being incomplete.
The most stable isotope of promethium, promethium-145, has a half-life of only 17.7 years, which makes it difficult to handle and study.
Its radioactivity also means that it can emit harmful radiation, making it potentially dangerous to handle without proper precautions.
Applications
Promethium has few practical applications due to its scarcity and high radioactivity. However, it can be used as a beta radiation source in nuclear batteries and in nuclear-powered spacecraft.
Additionally, it has been used in research to study the properties of metals and as a tracer in medical and industrial applications.
Interesting facts
Promethium is one of the rarest and most unstable elements on earth.
It has no stable isotopes, with its most stable isotope having a half-life of only 17.7 years.
The element was named after the Titan Prometheus from Greek mythology, who stole fire from the gods and gave it to humans.
Promethium was first discovered as a fission product of uranium fuel in nuclear reactors.
Due to its high radioactivity and short half-life, promethium has no commercial applications outside of scientific research.
Related
More elements
External links
- https://www.rsc.org/periodic-table/element/61/promethium
- https://en.wikipedia.org/wiki/Promethium
- https://www.britannica.com/science/promethium
- https://pubchem.ncbi.nlm.nih.gov/element/Promethium
- https://www.chemicool.com/elements/promethium.html
- https://www.livescience.com/38128-promethium.html
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
