
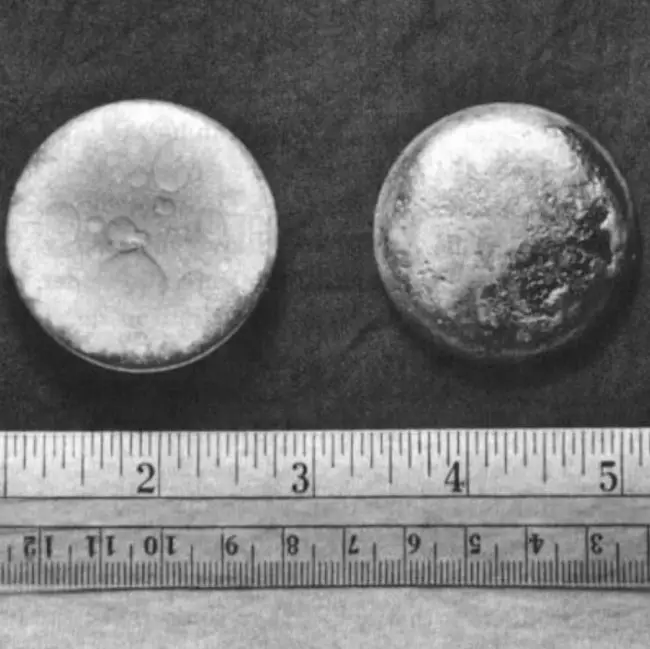
Plutonium (Pu) is a chemical element of the periodic table, located in the period 7, and has the atomic number 94. It is the sixth element in the actinide series. It is a bright, silvery-white metal that turns gray or yellow tarnish, when it is exposed to air. It is named after the planet Pluto. It is the second transuranium element and is counted as one of the radioactive elements.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – f block |
Plutonium is found in the actinide series, a group of elements located at the bottom of the periodic table. Specifically, in period 7, between neptunium (Np) and americium (Am).
Element information
 |
|
 |
|
| Origin of name | named after planet Pluto |
| Symbol | Pu |
| Atomic number (Z) | 94 |
| Atomic mass | (244) |
| Block | f-block |
| Period | 7 |
| Classification | Actinide |
| Atomic radius | 159 pm |
| Covalent radius | 187±1 pm |
| Melting point | 639.4 ℃, 1182.9 ℉, 912.5 K |
| Boiling point | 3228 ℃, 5842 ℉, 3505 K |
| Electron configuration | [Rn] 5f6 7s2 |
| Electrons per shell | 2, 8, 18, 32, 24, 8, 2 |
| Learn how to draw: Plutonium Bohr model | |
| Crystal structure | Monoclinic |
| Phase at r.t | Solid |
| Density near r.t | 19.85 g/cm3 |
| Natural occurrence | From decay |
| Oxidation state | +4 |
| Electronegativity (Pauling scale) | 1.28 |
| Protons Neutrons Electrons |
94 150 94 |
| CAS number | 7440-07-5 |
| Discovered by | Glenn T. Seaborg, Arthur Wahl, Joseph W. Kennedy, and Edwin McMillan in 1940-1941 |
History
Plutonium was first discovered by Glenn T. Seaborg, Edwin M. McMillan, Joseph W. Kennedy and Arthur C. Wahl in 1940. The discovery was made at the University of California, Berkeley, as part of a project to synthesize new elements by bombarding uranium with deuterium nuclei in a cyclotron.
The name “plutonium” was chosen to honor the dwarf planet Pluto, which had been discovered just a few years earlier. It also followed the tradition of naming new elements after planets – uranium, neptunium and plutonium were named after Uranus, Neptune and Pluto respectively.
During World War Ⅱ, the US government recognized the potential military uses of plutonium and initiated a secret program, the Manhattan Project, to produce enough plutonium to make atomic bombs. The first nuclear explosion, called Trinity, was a plutonium bomb, which was detonated in the desert of New Mexico in July 1945.
Occurrence and production
Plutonium is a radioactive element that is not found in nature in appreciable quantities. It is a product of nuclear reactions and can be produced in nuclear reactors or by bombarding uranium with neutrons in a particle accelerator. Plutonium is produced when uranium-238 absorbs a neutron and becomes uranium-239, which decays through a series of steps into plutonium-239.
The majority of plutonium is produced in nuclear reactors, where uranium-238 rods are bombarded with neutrons, and some of the uranium-238 is converted into plutonium-239. The plutonium-239 is then extracted from the spent fuel rods using a chemical process. Plutonium can also be produced by bombarding natural or enriched uranium with neutrons in a particle accelerator, a process known as neutron capture.
The production of plutonium is tightly controlled due to its potential use in nuclear weapons. The United States, Russia, and other countries with nuclear programs have extensive safety protocols in place to ensure the secure production and handling of plutonium.
Properties
Plutonium is a silvery-white metal that is highly radioactive and toxic.
It has a density that is significantly greater than that of lead and is one of the densest materials known.
It is a fissile material, meaning it can sustain a nuclear chain reaction, making it a valuable material for nuclear energy and weapons.
Plutonium has several allotropes, or different crystal structures, which can significantly affect its properties, such as its stability and behavior in different environments.
It is a reactive metal that can oxidize quickly in air, producing a layer of oxide that can be potentially dangerous if inhaled.
Plutonium-239 is the most common isotope used in nuclear weapons and energy production, while plutonium-238 is used in radioisotope thermoelectric generators (RTGs) for space exploration.
Applications
Nuclear weapons
Plutonium-239 is a key material in the production of nuclear weapons. The first nuclear bomb ever detonated, in 1945, used plutonium as its fissile material.
Nuclear power
Plutonium can be used as a fuel in nuclear reactors, both for power generation and for research purposes. Plutonium-239 is the most commonly used isotope in these applications.
Radioisotope thermoelectric generators
Plutonium-238 can be used as a power source in devices such as space probes and pacemakers. When the plutonium undergoes radioactive decay, it generates heat, which can be converted into electricity.
Research
Plutonium is used extensively in research on nuclear physics, nuclear chemistry, and materials science. Its unique properties make it an important tool for scientists studying a wide range of phenomena.
Neutron sources
Plutonium can be used as a neutron source in a variety of applications, including neutron radiography, activation analysis, and neutron scattering.
Target material
Plutonium can be used as a target material for the production of other isotopes, such as americium-241 and curium-242, which have a range of industrial and medical applications.
Interesting facts
Plutonium is named after the planet Pluto, which was discovered in 1930, just a few years before the element.
Plutonium-238 is used as a power source for space missions, such as the Mars rover and the Voyager spacecraft.
Plutonium-239 was used as the fissile material in the Fat Man atomic bomb dropped on Nagasaki, Japan, in 1945.
Plutonium-240 and other isotopes of plutonium are highly radioactive and can pose a serious health hazard if ingested or inhaled.
Plutonium-244 has the longest half-life of any plutonium isotope, over 80 million years.
Plutonium is so dense that it can ignite spontaneously at room temperature in the presence of air. This property makes it difficult to handle and store safely.
Related
More elements
External links
- https://www.rsc.org/periodic-table/element/94/plutonium
- https://en.wikipedia.org/wiki/Plutonium
- https://www.britannica.com/science/plutonium
- https://pubchem.ncbi.nlm.nih.gov/element/Plutonium
- https://www.chemicool.com/elements/plutonium.html
- https://www.space.com/what-is-plutonium
- https://www.ducksters.com/science/chemistry/plutonium.php
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
