
Americium (Am) is a chemical element of the periodic table, located in the period 7, and has the atomic number 95. It is the seventh element in the actinide series. It is a silvery-white metal which is named after America, where it was first made. It is the third transuranium element, highly toxic and is counted as one of the radioactive elements.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – f block |
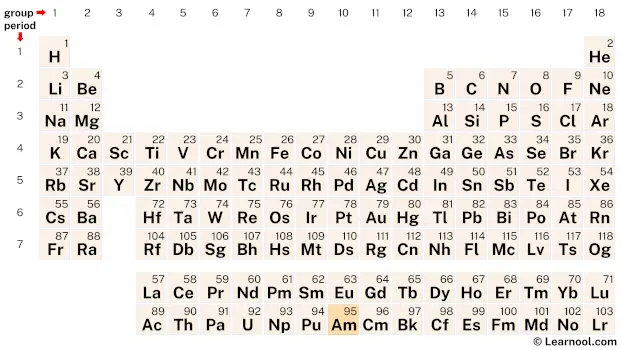
Americium (Am) is located on the periodic table in the actinide series, which is a group of elements located at the bottom of the table. Specifically, in period 7, between plutonium (Pu) and curium (Cm).
Element information
 |
|
 |
|
| Origin of name | named after America |
| Symbol | Am |
| Atomic number (Z) | 95 |
| Atomic mass | (243) |
| Block | f-block |
| Period | 7 |
| Classification | Actinide |
| Atomic radius | 173 pm |
| Covalent radius | 180±6 pm |
| Melting point | 1176 ℃, 2149 ℉, 1449 K |
| Boiling point | 2607 ℃, 4725 ℉, 2880 K (calculated) |
| Electron configuration | [Rn] 5f7 7s2 |
| Electrons per shell | 2, 8, 18, 32, 25, 8, 2 |
| Learn how to draw: Americium Bohr model | |
| Crystal structure | Double hexagonal close-packed (dhcp) |
| Phase at r.t | Solid |
| Density near r.t | 12 g/cm3 |
| Natural occurrence | Synthetic |
| Oxidation state | +3 |
| Electronegativity (Pauling scale) | 1.3 |
| Protons Neutrons Electrons |
95 148 95 |
| Learn how to find: Americium protons neutrons electrons | |
| CAS number | 7440-35-9 |
| Discovered by | Glenn T. Seaborg, Ralph A. James, Leon O. Morgan, and Albert Ghiorso in 1944 |
History
Americium was first produced in 1944 by a team of scientists led by Glenn T. Seaborg at the University of California, Berkeley. The team bombarded plutonium with neutrons in a nuclear reactor to produce americium-241, which was later separated and purified. The discovery of americium was part of a larger effort to synthesize new elements by bombarding heavy elements with neutrons, a technique known as nuclear transmutation.
The name “americium” was proposed by Seaborg and his team in honor of the Americas, as americium was the fourth transuranium element to be discovered by American scientists. Its discovery was also significant because it represented the first time that a new element had been intentionally produced in a laboratory. Americium has since been produced in small quantities for research purposes and for use in commercial applications.
In the years following its discovery, americium has become an important source of ionizing radiation for a variety of applications, particularly in the fields of nuclear medicine and industry. Its use in smoke detectors, which rely on the ionizing radiation emitted by americium-241 to detect smoke, has become one of its most well-known applications.
Occurrence and production
Americium is a man-made element and is not found in nature. The element is produced by bombarding plutonium-239 with neutrons in a nuclear reactor. Americium-241 is the most common isotope of americium and is produced by irradiating americium-240 or plutonium-239 with neutrons in a nuclear reactor. Americium-241 can also be produced by bombarding uranium-238 with neutrons to create plutonium-241, which can then decay into americium-241. Large quantities of americium-241 have been produced for use in smoke detectors and other applications. However, due to the hazardous nature of the element, production is tightly controlled.
Properties
Americium is a synthetic element and does not occur naturally on Earth.
It is a silvery-white metal and is similar in appearance to its neighboring elements, plutonium and neptunium.
Americium has a high radioactivity and emits alpha particles, beta particles, and gamma rays.
Its melting point is 1176 ℃, and its boiling point is approximately 2607 ℃.
Americium is a paramagnetic metal, meaning that it has a weak attraction to magnetic fields.
It has several oxidation states, with the most stable being +3 and +4.
Applications
Americium-241 is used in small amounts in household smoke detectors. It emits alpha particles which ionize the air, allowing a current to flow between two electrodes. If smoke enters the detector, it interrupts the current, triggering an alarm.
Americium-241 is used as a source of gamma radiation in industrial gauges for measuring the thickness of metal sheets, textiles, and paper.
Americium-241 is used as a gamma radiation source in radiography to check for flaws in metals, welds, and castings.
Americium-241 is used in nuclear research, as well as in the production of other radioactive isotopes.
Americium-241 is also used in the production of nuclear batteries for use in space probes and other remote locations where replacing batteries would be difficult.
Americium-241 can be used as a source of neutrons in nuclear weapons.
Interesting facts
Americium is the third synthetic element to be discovered and was named after the Americas, as it was first synthesized in the United States.
Americium-241 is used in many smoke detectors as it emits alpha particles, which ionize the air in the detector and trigger an alarm.
Americium is sometimes used in portable X-ray machines and in the oil industry to determine the porosity and permeability of rocks.
Americium-241 can be used in space exploration as a lightweight source of heat and electricity, as it generates significant amounts of heat through radioactive decay.
Americium-241 can also be used in gamma radiography to detect defects in metal welds and structures.
Americium-241 has a relatively long half-life of 432 years, which means that it remains radioactive for a long time.
Americium is highly toxic and can pose a serious health hazard if not handled properly, as it can accumulate in the bones and cause radiation damage.
Related
More elements
External links
- https://www.rsc.org/periodic-table/element/95/americium
- https://en.wikipedia.org/wiki/Americium
- https://www.britannica.com/science/americium
- https://pubchem.ncbi.nlm.nih.gov/element/Americium
- https://www.livescience.com/39874-facts-about-americium.html
- https://www.chemicool.com/elements/americium.html
- https://study.com/academy/lesson/americium-element-uses-in-everyday-life.html
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
