
Calcium (Ca) is a chemical element with the atomic number 20, that belongs to the group 2 and period 4 of the periodic table. It is a soft, silvery-white metal that belongs to the group of alkaline earth metals. In its pure form, it is relatively stable in air, but can react with water to form calcium hydroxide and hydrogen gas.[1] Calcium was first isolated in 1808 by Sir Humphry Davy in England. Its name originates from the Latin word “calx”, meaning lime, which is a compound of calcium.[2]
Calcium is the fifth most abundant element in the Earth’s crust and is primarily found in minerals such as limestone, gypsum, and fluorite. It is also present in the human body, with the majority of calcium stored in bones and teeth. Calcium is widely used in various industries, including construction, agriculture, and metallurgy. It is also an important component in the production of cheese and various other food products. Calcium’s unique properties make it a versatile and valuable element with a wide range of applications.
Calcium’s role in bone formation and maintenance is particularly important, as a deficiency in this element can lead to conditions such as osteoporosis. Calcium also plays a vital role in regulating muscle and nerve function, making it essential for proper heart and brain function. In addition to its importance in biology and industry, calcium has also been studied extensively by researchers for its potential health benefits. Some studies have suggested that calcium supplements may help reduce the risk of certain cancers, while others have linked calcium consumption to lower rates of hypertension and cardiovascular disease.[3][4]
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – s block |
Calcium is an s-block element, found in the second column and the fourth row of the periodic table. It has the atomic number 20 and is denoted by the symbol Ca.
Element information
 |
|
 |
|
| Origin of name | Latin word “calx” (which means lime) |
| Symbol | Ca |
| Atomic number (Z) | 20 |
| Atomic mass | 40.078 u |
| Block | s-block |
| Group | 2 |
| Period | 4 |
| Classification | Alkaline earth metal |
| Atomic radius | 197 pm |
| Covalent radius | 176±10 pm |
| Van der Waals radius | 231 pm |
| Melting point | 842 ℃, 1548 ℉, 1115 K |
| Boiling point | 1484 ℃, 2703 ℉, 1757 K |
| Electron configuration | [Ar] 4s2 |
| Electrons per shell | 2, 8, 8, 2 |
| Learn how to draw: Calcium Bohr model | |
| Crystal structure | Face-centered cubic (fcc) |
| Phase at r.t | Solid |
| Density near r.t | 1.55 g/cm3 |
| Main isotopes | Calcium-40, Calcium-42, Calcium-43, Calcium-44 |
| Natural occurrence | Primordial |
| Oxidation state | +2 |
| Electronegativity (Pauling scale) | 1.00 |
| Protons Neutrons Electrons |
20 20 20 |
| Learn how to find: Calcium protons neutrons electrons | |
| Valence electrons | 2 |
| Learn how to find: Calcium valence electrons | |
| CAS number | 7440-70-2 |
| Discovered by | Humphry Davy in 1808 |
History

In the late 1700s and early 1800s, Davy was conducting experiments on the decomposition of various substances using electricity.[5] In 1808, he turned his attention to lime (calcium oxide), which had been known since ancient times for its use in construction and as a fertilizer. Davy mixed lime with mercuric oxide and subjected the mixture to electrolysis, using a battery to generate an electric current. He observed a shiny, silvery metal collected at the negative electrode (cathode), which he identified as calcium.
Davy named the new element “calcium” after the Latin word for lime, “calx.” He published his discovery in a paper presented to the Royal Society in London in 1808. Davy’s discovery of calcium was an important milestone in the field of chemistry, as it was one of the first alkaline earth metals to be isolated. It also helped to establish the principles of electrolysis and electrochemistry, which would later play a crucial role in the development of modern chemistry and technology. Today, calcium is an essential element for life and is used in a wide range of applications, from building materials to dietary supplements. The discovery of calcium by Sir Humphry Davy paved the way for a greater understanding of the properties and uses of this important element.
Occurrence and production
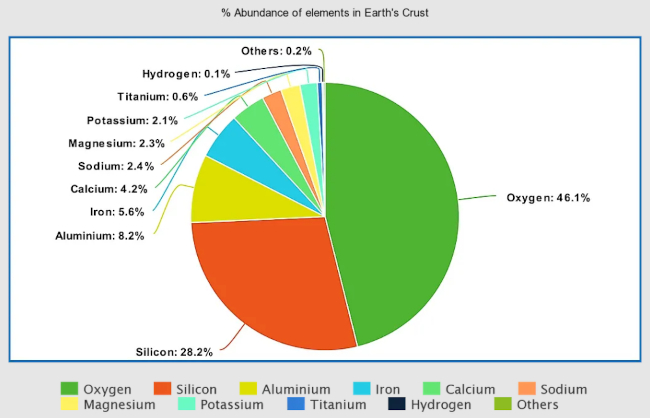
Calcium is one of the most abundant elements in the Earth’s crust, making up about 4% of the crust by weight. It is also one of the most abundant elements in the human body, where it is found in bones and teeth. In addition to its presence in the Earth’s crust and in biological materials, calcium is also found in the oceans, where it is dissolved in the water. It is estimated that the oceans contain about 35,000 billion metric tons of dissolved calcium.
Calcium is primarily obtained through the mining of calcium-containing minerals, such as limestone, dolomite, gypsum, and fluorite. These minerals are typically extracted through mining operations and then processed to obtain calcium in its pure form. In addition, calcium can also be produced through a variety of chemical and physical methods, including electrolysis of calcium chloride or calcium fluoride, and thermal reduction of calcium oxide with aluminum.
Several countries are major producers of calcium-containing minerals. China is the world’s largest producer of calcium carbonate, which is used in a wide range of applications, including paper, plastics, and construction materials. Other major producers of calcium-containing minerals include the United States, Russia, India, and Japan. In addition to these countries, many other nations also have significant reserves of calcium-containing minerals, highlighting the widespread availability of this important element.
Properties
Calcium is a soft, silvery-white, alkaline earth metal that is solid at room temperature.
It has a melting point of 842 ℃ and a boiling point of 1484 ℃, and a density of 1.55 g/cm³, which is less than that of aluminum.
Calcium has a face-centered cubic crystal structure, and a Mohs hardness of 1.5.
It is a good conductor of both electricity and heat, with high electrical and thermal conductivity compared to other metals.
Calcium is paramagnetic, weakly attracted to a magnetic field.
It is highly reactive and quickly forms a thin layer of calcium oxide on its surface when exposed to air, which protects the metal from further oxidation.
Calcium has relatively low reactivity with water compared to other alkaline earth metals, and will slowly react with water to form calcium hydroxide and hydrogen gas.
It is essential for living organisms, as it is a key component of bones and teeth, and is involved in many biological processes, such as muscle contraction, nerve function, and blood clotting.
Calcium also has a role in plant growth and development, and is found in many fertilizers.
Calcium compounds have a variety of uses, such as in the production of cement, glass, and ceramics, and as a reducing agent in the extraction of metals from ores.
Applications
Construction and architecture
Calcium is an essential ingredient in cement, mortar, and concrete, which are the building blocks of modern infrastructure. It provides strength and durability to these materials and helps them withstand the test of time, making them suitable for use in large-scale construction projects such as bridges, buildings, and roads.
Agriculture
Calcium is an essential micronutrient for plant growth and development. It helps in the formation and maintenance of strong cell walls, which are crucial for plant structure and support. Calcium deficiency can lead to stunted growth, reduced yield, and poor crop quality. As a result, calcium is often added to soil as a fertilizer to improve crop yield and quality.
Health and nutrition
Calcium is an important mineral for human health. It plays a vital role in maintaining strong bones and teeth, regulating muscle function, supporting nerve function, and aiding in blood clotting. Adequate calcium intake is crucial for people of all ages to maintain good health, and calcium supplements are often recommended for those who don’t get enough calcium from their diet.
Metallurgy
Calcium is used as a reducing agent in the production of metals such as uranium, zirconium, and thorium. This process involves the reduction of metal oxide using calcium, resulting in the formation of metal and calcium oxide. The calcium oxide is then used in the production of cement.
Pharmaceuticals
Calcium is used as a supplement in the form of calcium carbonate or calcium citrate to treat and prevent calcium deficiency, as well as to reduce the risk of osteoporosis. It is also used in some medications to treat conditions such as heartburn, acid indigestion, and high blood pressure.
Water treatment
Calcium is commonly used in water treatment to remove impurities and soften hard water. Hard water can cause scaling and other problems in pipes and equipment, which can lead to increased maintenance costs and decreased efficiency. Calcium helps to remove these impurities and prevent scaling, ensuring that water is safe and suitable for use in various industries.
Pyrotechnics

Calcium is a key ingredient in many pyrotechnic devices, including fireworks, flares, and other visual effects. When heated, calcium produces bright orange-red flames, making it a popular choice for creating visually stunning displays.
Interesting facts
Calcium, being the fifth most abundant element in the Earth’s crust, is found in rocks, minerals, and soils. It is also present in the oceans and is an important component of marine life.
Calcium is a crucial nutrient for human health, playing a vital role in maintaining strong bones and teeth, regulating muscle function, supporting nerve function, and aiding in blood clotting.
While dairy products are commonly associated with calcium, there are many other food sources of this essential mineral, including leafy green vegetables, nuts and seeds, beans and lentils, and fortified foods such as cereals and orange juice.
Calcium has been used as a building material since ancient times, with the ancient Romans using it to construct buildings and roads.
Calcium can react violently with water, which is why it is never found in its pure form in nature.
Calcium also plays a role in regulating the body’s pH levels, as it can neutralize acids and help maintain a proper acid-base balance.
Some studies suggest that calcium may have a role in reducing the risk of certain cancers, such as colon cancer, although more research is needed to confirm this.
Calcium is also important for cardiovascular health, as it can help regulate blood pressure and reduce the risk of heart disease.
Calcium deficiency can lead to a range of health problems, including osteoporosis, muscle cramps, and even seizures.
In addition to its many health benefits, calcium also has industrial applications, such as being used in the production of cement, steel, and other metals.
Related
More elements
References
1. Calcium Metal Reactivity with Water – UMass Amherst
2. Calcite (Calcium Carbonate) – Ohio History Central
4. Can calcium supplementation reduce the risk of high blood pressure?
5. Davy’s Elements (1805-1824) – University of Waterloo
External links
- https://en.wikipedia.org/wiki/Calcium
- https://www.rsc.org/periodic-table/element/20/calcium
- https://www.britannica.com/science/calcium
- https://pubchem.ncbi.nlm.nih.gov/element/Calcium
- https://www.chemicool.com/elements/calcium.html
- https://www.thoughtco.com/calcium-element-facts-p2-606512
- https://www.ducksters.com/science/chemistry/calcium.php
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
