Alkaline earth metals are a group of chemical elements comprising beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). These metals are found in the second column of the periodic table, also known as group 2 elements. The term “alkaline earth” was first used by the French chemist Antoine Lavoisier in the late 18th century, and it was coined because these elements were found to form alkaline solutions when they reacted with water.
The name “alkaline” was given to these metals because of their basic nature, which is similar to that of alkali metals. However, the two groups of metals are quite different from each other in terms of their reactivity, physical and chemical properties. The alkaline earth metals have relatively high melting and boiling points compared to alkali metals, and they are also less reactive.
The alkaline earth metals were first discovered in the late 18th century by a number of scientists, including Humphry Davy, who isolated the elements magnesium, calcium, strontium, and barium by means of electrolysis. Beryllium was first isolated by Friedrich Wöhler and Antoine Bussy in 1828 by the reduction of beryllium chloride with potassium.
Alkaline earth metals are important because of their wide range of uses in various fields. For example, magnesium and calcium are essential for biological processes in plants and animals, while beryllium is used in nuclear reactors and X-ray tubes. Strontium and barium compounds are used in the production of pyrotechnics and fireworks, and barium is also used in medical imaging tests to visualize the gastrointestinal tract. Radium, which is a radioactive element, was once used in luminous paints for watches and instrument dials, but its use has been largely discontinued due to its harmful health effects.
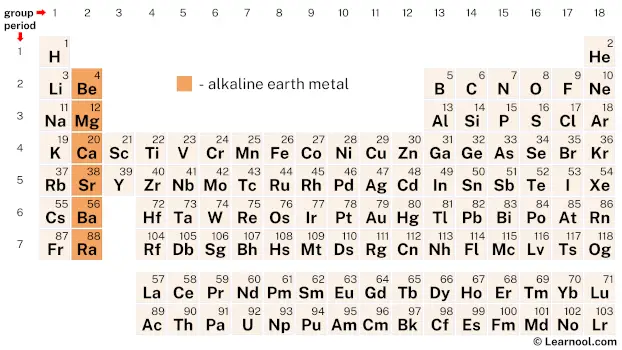
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – alkaline earth metal |
Alkaline earth metals are a group of six chemical elements located in group 2 of the periodic table, which includes beryllium, magnesium, calcium, strontium, barium, and radium.
History

The use of alkaline earth metals dates back to ancient times when people used materials such as limestone and gypsum for building purposes. These materials contain calcium carbonate and calcium sulfate, respectively, which are examples of alkaline earth compounds.
Beryllium and magnesium were the first alkaline earth metals to be discovered in modern times. In 1798, French chemist Louis Nicolas Vauquelin discovered beryllium while analyzing beryl and emeralds. He named the new element after the mineral’s name, beryl. Magnesium was discovered in 1808 by Sir Humphry Davy through the electrolysis of magnesium oxide.
Calcium and strontium were discovered by two different scientists around the same time. In 1808, Sir Humphry Davy discovered calcium through the electrolysis of lime. In 1790, a Scottish physician named Adair Crawford and his colleague William Cruickshank discovered strontium in the mineral strontianite, which was named after the Scottish village where it was found.
Barium was discovered by Sir Humphry Davy in 1808 through the electrolysis of molten baryta (barium oxide). Radium, the last element in the alkaline earth metal series, was discovered in 1898 by Marie and Pierre Curie in pitchblende, a uranium-rich ore. The Curies isolated radium in its metallic form through the purification of radium chloride.
These discoveries opened up new possibilities for research and practical applications of these elements. In the following years, scientists explored their properties, and the alkaline earth metals became an essential part of various industries, such as agriculture, construction, and medicine.
Occurrence and production
Beryllium and magnesium occur naturally in a variety of minerals. Beryllium is found in beryl and bertrandite, while magnesium is found in minerals such as magnesite, dolomite, and carnallite. Beryllium has a concentration of about 2-6 parts per million (ppm), making it a relatively rare element, while magnesium is the eighth most abundant element in the Earth’s crust, constituting about 2% of its weight. The largest producers of beryllium are the United States, China, and Kazakhstan. The largest producers of magnesium are China, Russia, and Turkey.
Beryllium is usually produced by reducing beryllium fluoride with magnesium metal. The reduction process is followed by a series of chemical reactions to obtain beryllium metal. The production of magnesium involves the electrolysis of magnesium chloride, which is obtained from seawater or brine deposits.
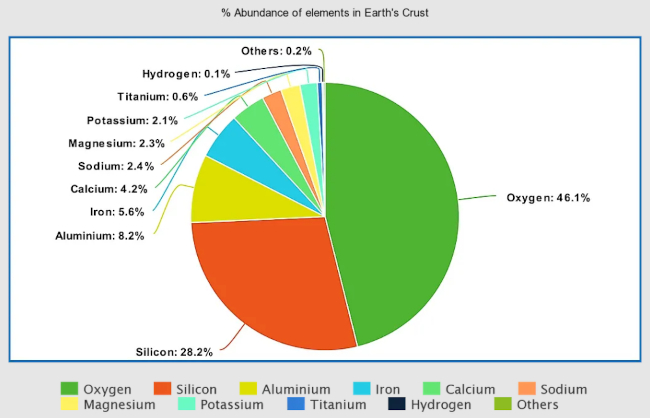
Calcium and strontium are also widely distributed in the Earth’s crust and can be found in minerals such as limestone, gypsum, celestine, and strontianite. Calcium is the fifth most abundant element in the Earth’s crust, with a concentration of about 4% by weight, while strontium is the fifteenth most abundant element in the Earth’s crust, with a concentration of about 360 parts per million. The largest producers of calcium are China, Russia, and the United States, while Mexico, Spain, and Argentina are the largest producers of strontium.
Barium and radium occur in very small quantities in the Earth’s crust. Barite and witherite are the main minerals that contain barium, while radium is found in uranium ores such as pitchblende. Barium is the 14th most abundant element in the Earth’s crust, making up about 0.05% of its weight, while radium is extremely rare, comprising only 1 part per trillion of the Earth’s crust. The largest producers of barium are China, India, and Morocco. Radium, however, is not produced commercially and is only obtained as a byproduct of uranium and thorium processing.
Properties
Physical properties
Alkaline earth metals are shiny, silvery-white metals.
They are soft and have low densities.
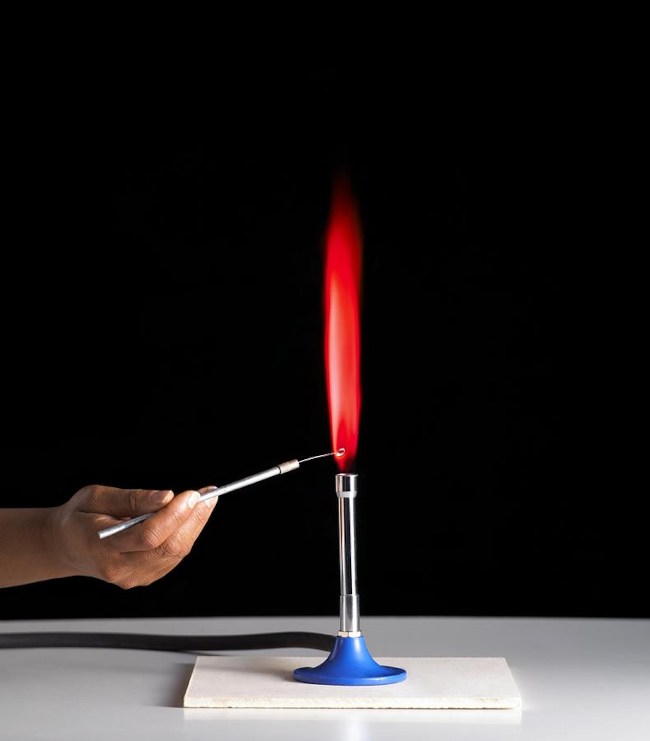
Alkaline earth metals are highly reactive and can be identified by their flame colors when heated with a Bunsen burner. For example, calcium produces a brick-red flame, strontium produces a crimson flame, and barium produces an apple-green flame.
They are good conductors of heat and electricity.
The melting and boiling points of alkaline earth metals are generally higher than those of alkali metals.
Chemical properties
All alkaline earth metals, with the exception of beryllium, react with water.
They have a +2 oxidation state, meaning they readily lose two electrons to form positive ions.
The electronegativity values of alkaline earth metals range from about 0.9 to 1.5, making them relatively electropositive and likely to lose electrons easily.
Alkaline earth metals are highly reactive with nonmetals such as oxygen and chlorine, and tend to form ionic compounds with them.
Other properties
Alkaline earth metals generally have higher ionization energies compared to alkali metals. This is because alkaline earth metals have a greater number of electrons in their outermost energy level, making it harder to remove an electron from them.
The alkaline earth metals have smaller atomic radii compared to those of the corresponding alkali metals.
Trends
Atomic radius
As you move down the group, the atomic radius of the alkaline earth metals increases. This is due to the addition of new electron shells as you move down the group, which results in an increase in the distance between the nucleus and the outermost electrons.
Ionization energy
Ionization energy is the energy required to remove an electron from an atom or ion. As you move down the group, the ionization energy of the alkaline earth metals decreases. This is due to the increased shielding effect provided by the additional electron shells, which reduces the attraction between the nucleus and the outermost electrons.
Electronegativity
Electronegativity is the measure of an atom’s ability to attract electrons towards itself in a chemical bond. As you move down the group, the electronegativity of the alkaline earth metals decreases. This is due to the increased atomic radius and shielding effect, which reduces the attraction between the nucleus and the outermost electrons, making it more difficult for the atoms to attract additional electrons.
Melting and boiling points
As you move down the group, the melting and boiling points of the alkaline earth metals decrease. This is due to the increased atomic radius and shielding effect, which results in weaker metallic bonding between the atoms, making it easier for the atoms to move past one another and transition from a solid to a liquid or gas state.
Reactivity
The reactivity of alkaline earth metals increases as you go down the group due to a decrease in ionization energy and an increase in atomic radius. The decrease in ionization energy makes it easier for the outermost electrons to be removed, while the increase in atomic radius leads to a decrease in effective nuclear charge, making it easier for the outermost electrons to be involved in chemical reactions. This makes the alkaline earth metals more reactive down the group.
Applications
Alloys
Alkaline earth metals are used to produce a variety of alloys, such as magnesium-aluminum, beryllium-copper, and others. These alloys exhibit unique properties such as high strength, resistance to corrosion and heat, making them useful in the production of parts for aerospace and automotive industries.
Construction materials
Calcium and magnesium are commonly used in the production of cement and concrete, due to their ability to enhance the compressive strength and durability of the material. Magnesium is also used as a fire retardant in construction materials.
Medicine
Calcium is an essential nutrient in the human body and is used in the production of medicines such as antacids and calcium supplements. Barium and strontium are used in medical imaging techniques such as barium meals and bone scans.
Lighting

Strontium and barium are used in the production of red and green fireworks, respectively. Strontium is also used in the production of cathode-ray tubes for televisions and computer monitors.
Agriculture
Magnesium is an important nutrient for plant growth and is used as a fertilizer in agriculture. Calcium is also used in the production of fertilizers and can help improve soil structure.
Other applications
Beryllium is used in the production of nuclear reactors and aerospace components, while radium was once used in luminous paints and dials, although its use has been largely discontinued due to its radioactivity.
Interesting facts
The name “alkaline earth” comes from the fact that the oxides of these metals are alkaline in nature.
Alkaline earth metals have lower densities and are relatively soft compared to most metals, but they have higher melting points than alkali metals.
All alkaline earth metals have two valence electrons, making them highly reactive.
Beryllium is the lightest of all alkaline earth metals and has a very high melting point. It is a rare element that is found in only a few minerals.
Magnesium is used in the production of magnesium oxide, which is used as a refractory material in the steel industry.
Calcium is essential for the formation of bones and teeth and is also used in the production of cement, cheese, salmon, and various other products.
Strontium is used in the production of flares and fireworks, as it produces a bright red flame when burned. It is also used in the production of ferrite magnets, which are used in electronic devices.
Barium is used in the production of vacuum tubes and other electronic devices. One of its compounds, barium sulfate, is commonly used in medical imaging procedures such as X-rays and CT scans.
Radium was discovered by Marie and Pierre Curie in 1898 and was once used as a treatment for cancer. It is a highly radioactive element that was once used in luminous paints and watches.
Related
More topics
- Block (periodic table)
- Group (periodic table)
- Period (periodic table)
- Metal
- Alkali metal
- Alkaline earth metal
- Lanthanide
- Actinide
- Transition metal
- Post-transition metal
- Metalloid
- Nonmetal
- Reactive nonmetal
- Noble gas
External links
- Alkaline-earth metal | Properties, List, & Reactivity – Britannica
- Alkaline earth metal – Wikipedia
- 6.10: Alkaline Earth Metals – Chemistry LibreTexts
- Group 2A — The Alkaline Earth Metals – Angelo State University
- Alkaline Earth Metals – Periodic Table – ChemTalk
- Alkaline Earth Metals Their Chemical Characteristics – Jack Westin
- Alkaline Earth Metals – CK-12
- Alkaline Earth Metals | Definition, Properties & Effects – Study.com
- Alkaline Earth Metals – Elements & Periodic Table – Chem4Kids
- Alkaline Earth Metal – an overview – ScienceDirect
- What Are the Properties of the Alkaline Earth Metals? – ThoughtCo
- Periodic Table of the Elements – Alkaline Earth Metals – Cool Periodic Table
- The Periodic Table – Chemistry – UH Pressbooks
- Alkaline Earth Metals – Diamond Light Source
- Alkaline-earth metal Definition & Meaning – Dictionary.com
- Alkaline-Earth Metals on the Periodic Table – Chemistry Learner
- Alkali and Alkaline Earth Metals – TechnologyUK
- Chemistry for Kids: Elements – Alkaline Earth Metals – Ducksters
- Alkaline-earth Metals – Encyclopedia.com
- Alkaline earth metal Definition & Meaning – Merriam-Webster
- ALKALINE EARTH METAL definition – Cambridge Dictionary
- Alkaline Earth Metals – Fisher Scientific
- Alkaline Earth Metals — Overview & Properties – Expii
- Information on Alkali Metals – Stanford Environmental Health & Safety
- The Alkaline Earth Metals | Introduction to Chemistry – Course Hero
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.






