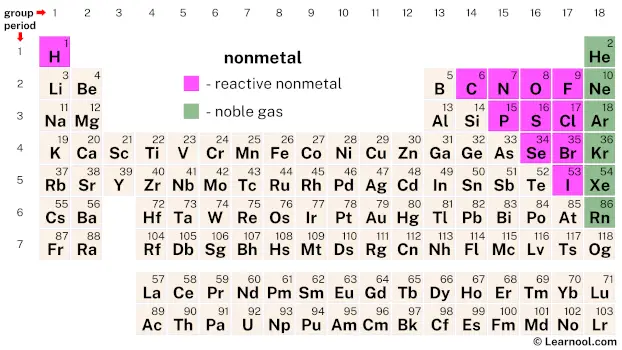
Nonmetals are a group of elements that have very different properties from metals. They are found in the upper right-hand corner of the periodic table and include 17 elements, with hydrogen, carbon, nitrogen, oxygen, fluorine, and chlorine being some of the most well-known. In addition to these reactive nonmetals, there are also noble gases such as helium, neon, and argon that are considered nonmetals.
Nonmetals are characterized by their generally low melting and boiling points and exhibit distinct properties in comparison to metals. They are often gases or brittle solids at room temperature, rather than the shiny, malleable solids that we associate with metals. Nonmetals are also poor conductors of heat and electricity, which is in contrast to metals, which are generally good conductors.
In addition to their physical properties, nonmetals also have very different chemical properties from metals. For example, nonmetals tend to form covalent bonds, in which they share electrons with other atoms to form molecules. This is in contrast to metals, which tend to form metallic bonds, in which they share electrons in a lattice structure. Nonmetals can also be very reactive, often reacting with metals to form ionic compounds.
Nonmetals, despite being relatively scarce in the Earth’s crust, are essential components of many important molecules and compounds. Carbon is the basis of all known life on Earth, while oxygen is essential for respiration and nitrogen is a key component of DNA and proteins. Nonmetals also have important industrial uses, such as in the production of fertilizer, plastics, and electronics. The properties and behavior of nonmetals are therefore important to understand and crucial, particularly in fields such as chemistry, biology, and materials science.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – reactive nonmetal | |||
| – noble gas | |||
There are a total of 17 nonmetals on the periodic table, including reactive nonmetals like hydrogen, carbon, nitrogen, oxygen, fluorine, phosphorus, sulfur, chlorine, selenium, bromine, and iodine, as well as noble gases such as helium, neon, argon, krypton, xenon, and radon.
History

The discovery of nonmetals can be traced back to the 1600s when several elements were discovered. Phosphorus was discovered in 1669 by German alchemist Hennig Brand. Carbon was also known in ancient times, but its nature as an element was not recognized until the 18th century. Hydrogen, a nonmetal and the lightest element, was discovered in 1766 by English chemist Henry Cavendish. Nitrogen was discovered by Scottish physician Daniel Rutherford in 1772, and oxygen was discovered independently by Swedish chemist Carl Wilhelm Scheele and English clergyman Joseph Priestley in the late 1700s.
Throughout the following centuries, several additional nonmetals were discovered. Sulfur was known to the ancient Greeks and Romans. Chlorine was discovered in 1774 by Swedish chemist Carl Wilhelm Scheele and was given its current name by English chemist Sir Humphry Davy in 1810. It was named after its yellow-green color. Other nonmetals discovered in the 19th century include selenium and bromine.
In the late 19th century, several noble gases were discovered that are part of the nonmetals group. Helium was discovered in 1868 by French astronomer Jules Janssen and British astronomer Joseph Norman Lockyer. Neon was discovered in 1898 by British chemists Sir William Ramsay and Morris Travers. Argon was discovered in 1894 by Ramsay and British chemist Lord Rayleigh. These elements were originally referred to as the “inert gases” due to their lack of reactivity.
The remaining nonmetals, including fluorine, krypton, xenon, and radon, were discovered in the 19th century. Fluorine was discovered in 1886 by French chemist Henri Moissan, while krypton and xenon were discovered in 1898 by Ramsay and Travers. Radon, the heaviest of the noble gases and a radioactive element, was discovered in 1899 by German physicist Friedrich Ernst Dorn. Selenium, which was originally discovered in the 19th century, was also found to have semiconducting properties in the 20th century and is used in modern electronics.
Occurrence and production
Nonmetals are generally found in the Earth’s crust and atmosphere, as well as in living organisms. Some nonmetals, such as oxygen and nitrogen, make up a significant portion of the Earth’s atmosphere. Other nonmetals, such as sulfur and phosphorus, are found in minerals and rocks in the Earth’s crust. Some nonmetals, such as carbon, are found in both living and nonliving things.
While nonmetals are generally less abundant in the Earth’s crust than metals, some are still present in significant amounts. For instance, oxygen, the most abundant element on Earth, comprises about 46-47% of the Earth’s crust by mass. Nitrogen, another nonmetal, is also relatively abundant, accounting for about 78% of the Earth’s atmosphere by volume. Sulfur, on the other hand, is found in minerals like pyrite, gypsum, and cinnabar, and it ranks as the tenth most abundant element in the universe.
Nonmetals can be produced in various ways, depending on the element. Hydrogen, for instance, can be produced through electrolysis, a process that involves passing an electric current through water to break apart the hydrogen and oxygen molecules. Other nonmetals, like chlorine and fluorine, can be produced through chemical reactions. Carbon, on the other hand, is a naturally occurring element found in soil from dead and decaying animals, as well as animal waste. Marine organisms, ranging from marsh plants and fish to seaweed and birds, also produce carbon through their life and death.
Properties
Physical properties
Nonmetals have several physical properties that distinguish them from metals. For instance, nonmetals are typically poor conductors of heat and electricity. This means they do not allow electric currents or thermal energy to flow through them easily. Nonmetals have low melting and boiling points, and most are gases or brittle solids at room temperature. They are also typically dull and lack the luster and shine of metals.
Chemical properties
Nonmetals have different chemical properties than metals and they tend to form covalent bonds by sharing electrons with other atoms to form molecules, whereas metals tend to form metallic bonds in which they share electrons in a lattice structure. Nonmetals are also more electronegative than metals, meaning they have a greater attraction for electrons. This makes nonmetals react vigorously with metals, and they often form ionic compounds.
Reactivity
Nonmetals vary widely in their reactivity. Some nonmetals, such as the noble gases, are inert and do not readily react with other elements. Others, such as halogens like fluorine and chlorine, are highly reactive and can form compounds with most other elements. Nonmetals can also form acids when they react with water, which can then react with metals to form salts.
Allotropes
Many nonmetals exist in different forms, or allotropes. For instance, carbon can exist as diamond, graphite, or fullerenes, all of which have very different physical and chemical properties. Oxygen can exist as O2, which is a colorless gas, or O3, which is a blue gas with a distinct odor. The properties of allotropes can vary significantly, even though they are made up of the same element.
Applications
Fertilizers
Nonmetals such as nitrogen, phosphorus, and sulfur are essential components of fertilizers, which are used to enhance plant growth and increase crop yields. Nitrogen is particularly important as it is a key component of amino acids, which are the building blocks of proteins in plants and animals.
Plastics
Many plastics are made from nonmetallic elements, such as carbon and hydrogen. For example, polyethylene, which is used in plastic bags and packaging materials, is made from repeating units of ethylene, a molecule composed of two carbon atoms and four hydrogen atoms.
Medicine
Nonmetals have important roles in medicine as well. For example, iodine is used in thyroid hormones, which regulate metabolism in the body, and chlorine is used in disinfectants to kill bacteria and other microorganisms.
Lighting

Nonmetals such as neon, argon, and xenon are used in gas-discharge lamps, which are commonly used in advertising signs, vehicle headlights, and other applications where bright, colorful lighting is desired.
Interesting facts
Carbon is the only nonmetal that can form long chains and rings, which are essential for the formation of complex organic molecules like proteins, DNA, and carbohydrates, making it the basis of all known life on Earth.
The most abundant nonmetal on Earth is oxygen, making up around 21% of the atmosphere.
Nonmetal fluorine is the most electronegative element, meaning it has the highest ability to attract electrons to itself.
Chlorine gas was used as a weapon in World War Ⅰ and caused many casualties due to its poisonous nature.
Nonmetals tend to be brittle, meaning that they are prone to breaking or shattering when force is applied, unlike metals which are malleable and can be bent without breaking.
Bromine is the only nonmetal that is liquid at room temperature.
The density of helium is so low that it can make balloons float.
Radon, a radioactive gas, is a leading cause of lung cancer.
Related
More topics
- Block (periodic table)
- Group (periodic table)
- Period (periodic table)
- Metal
- Alkali metal
- Alkaline earth metal
- Lanthanide
- Actinide
- Transition metal
- Post-transition metal
- Metalloid
- Nonmetal
- Reactive nonmetal
- Noble gas
External links
- Nonmetal – Wikipedia
- The Chemistry of Nonmetals – Purdue University
- Metals, Metalloids, and Nonmetals – Angelo State University
- Nonmetal | Definition, Properties, Examples, & Facts – Britannica
- Nonmetals Definition and Properties – ThoughtCo
- Nonmetals – CK-12
- Periodic Table Metals and Non-Metals – ChemTalk
- Non-Metals – Chemical Elements.com
- Characteristics of Nonmetals – Chemistry LibreTexts
- What’s the Difference Between Metals, Nonmetals, and Metalloids? – Mead Metals
- Chemistry for Kids: Elements – Nonmetals – Ducksters
- Lesson Explainer: Metals, Nonmetals, and Metalloids – Nagwa Limited
- Metals and Nonmetals – HyperPhysics Concepts
- Lesson 3.4 Nonmetals and Metalloids – Pleasant Valley School District
- Structure and General Properties of the Nonmetals – UH Pressbooks
- Nonmetal Elements | Definition, Properties & Examples – Study.com
- Nonmetal – an overview – ScienceDirect
- Metals and Non-metals: Examples & Definition – Vaia
- Nonmetal – New World Encyclopedia
- The Periodic Table: Metals, Nonmetals, and Metalloids – Dummies
- Nonmetal – Encyclopedia.com
- Tricks for Knowing Locations of Metals and Nonmetals on Periodic Table – Laurence Lavelle
- What are metals and non-metals on the periodic table? – BBC
- Metals vs Nonmetals – Science Notes and Projects
- Nonmetal – Chem Europe
- Nonmetals – Chemistry Learner
- Metals, Nonmetals, & Metalloids – Window to the Universe
- Metals and Nonmetals – Chemistry – Socratic
- Periodic Table of the Elements – Other Nonmetals – Cool Periodic Table
- Non-Metals – Diamond Light Source
- Nonmetal – Definition, Meaning & Synonyms – Vocabulary.com
- Chemistry / Characteristics of Metals and Nonmetals – North Polk Community School District
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
