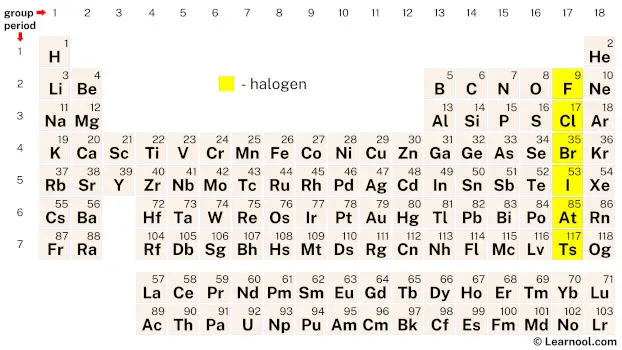
Halogens, also known as the fluorine group, are a group of elements located in group 17 of the periodic table. This group comprises six chemical elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). While the first five elements are widely accepted as halogens due to their shared properties, the classification of tennessine as a halogen is still a subject of debate among scientists. Positioned in the p-block, the halogens possess seven valence electrons, making them highly reactive and eager to gain one additional electron to achieve a stable octet configuration.
Among the halogens, fluorine is the lightest element in the group. Four elements in this group, namely fluorine, chlorine, bromine, and iodine, are classified as reactive nonmetals due to their tendency to readily form negative ions. Astatine, on the other hand, is a relatively rare element and is categorized as a post-transition metal. The final element in the halogen group is tennessine, which is a synthetic element that is not naturally occurring.
The term “halogen” was coined by the Swedish chemist Jöns Jacob Berzelius in the early 19th century. The word is derived from Greek roots, with “halo” meaning “salt” and “gen” meaning “former” or “maker.” This name reflects the halogens’ remarkable property of readily combining with metals to form salts. When halogens react with metals, they undergo a process known as halogenation, resulting in the formation of various metal halides. Beyond their reactivity with metals, halogens have diverse applications. For example, chlorine is widely used for water disinfection, bromine finds use in flame retardants, and iodine is utilized in antiseptics and as a nutrient in the human body.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – halogen |
On the periodic table, the halogens are a group of highly reactive chemical elements. This group comprises fluorine, chlorine, bromine, iodine, astatine, and tennessine.
Related
More topics
External links
- Halogen | Elements, Examples, Properties, Uses, & Facts – Britannica
- Halogen – Wikipedia
- The Chemistry of the Halogens – Purdue University
- 6.12: Halogens – Chemistry LibreTexts
- Halogen Elements and Properties – ThoughtCo
- Halogens – Periodic Table – ChemTalk
- Halogens – Elements & Periodic Table – Chem4Kids
- Occurrence, Preparation, and Properties of Halogens – UH Pressbooks
- Halogens – humans, body, used, water, process, Earth, life, plants – Science Clarified
- Halogens – Chemical Elements.com
- Group 7A — The Halogens – Angelo State University
- The Halogens – University Science Books
- Halogens and Noble Gases – Periodic Table – Turito
- Halogens – Chemistry for Kids: Elements – Ducksters
- Halogens Their Chemical Characteristics – Jack Westin
- Halogen Definition & Meaning – Merriam-Webster
- Halogen Elements – List and Facts – Science Notes and Projects
- Halogens | Facts & Definition – A-Level Chemistry
- Halogens – Chemistry Learner
- Halogens of the Periodic Table | Properties, Reactivity & Uses – Video & Lesson Transcript – Study.com
- Halogens – Chemistry Encyclopedia – uses, elements, gas, number, name, symbol, salt, atom – Chemistry Explained
- Halogen Definition & Meaning – Dictionary.com
- Halogens – Course Hero
- Halogens – Concept – Chemistry Video – Brightstorm
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.




