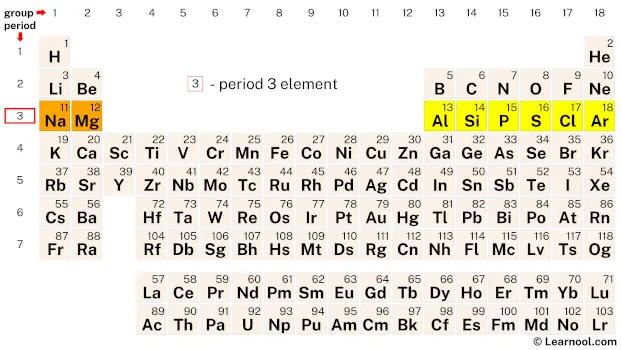
Period 3 elements are the set of chemical elements found in the third row or period of the periodic table. This row contains eight elements: sodium (Na), magnesium (Mg), aluminum (Al), silicon (Si), phosphorus (P), sulfur (S), chlorine (Cl), and argon (Ar). These elements possess a wide range of physical and chemical properties, which make them essential for a multitude of applications in various fields. Most p-block elements in period 3 can have expanded octets and hold more than 8 electrons in their valence shell, with the exception of aluminum which can have less than 8 electrons. Elements silicon and onward are the only ones that can have more than 8 electrons.
Period 3 elements display a wide range of properties and applications. Sodium and magnesium are both lightweight metals with low densities, while aluminum is a lightweight metal with a higher density. Silicon is a metalloid and is widely used in the production of electronic components. Phosphorus and sulfur are nonmetals, and while phosphorus is an essential element for life, sulfur has a wide range of industrial applications. Chlorine is a highly reactive gas used in the production of various chemicals, while argon is a noble gas with a low reactivity and is used in welding and lighting applications.
Period 3 elements demonstrate several trends in their physical and chemical properties, such as atomic size, ionization energy, and electronegativity. For example, as we progress across period 3 from left to right, the atomic size decreases due to the increased nuclear charge. As a result, this leads to higher ionization energies. This trend is clearly illustrated by the transition from sodium, with a larger atomic size and lower ionization energy, to chlorine, with a smaller atomic size and higher ionization energy.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| 3 | – period 3 element |
Period 3 on the periodic table contains eight elements, namely sodium, magnesium, aluminum, silicon, phosphorus, sulfur, chlorine, and argon.
Element information
Sodium

Sodium is a soft, silvery-white metal that is highly reactive with water and air. It is commonly found in nature as part of various minerals, such as halite and trona. Sodium has a relatively low melting and boiling point compared to other metals, melting at 97.79 ℃ (208.02 ℉) and boiling at 882.94 ℃ (1621.29 ℉). It is an important element in many industries, including the production of chemicals, soap, and glass.
Magnesium

Magnesium is a silvery-white metal that is highly reactive, but not as reactive as sodium. It is a relatively abundant element in the Earth’s crust, and is commonly found in minerals such as dolomite and magnesite. Magnesium has a higher melting and boiling point compared to sodium, melting at 650 ℃ (1202 ℉) and boiling at 1091 ℃ (1994 ℉). Magnesium is used in a variety of applications, such as in the production of alloys, in pyrotechnics, and as a component of flares and incendiary devices.
Aluminum

Aluminum is a silvery-white, lightweight metal with atomic number 13. It is the third most abundant element in the Earth’s crust, and its compounds are widely used in a variety of applications, such as construction, transportation, and packaging. Aluminum is highly reactive, and forms a protective oxide layer on its surface that makes it resistant to corrosion. Its atomic radius is smaller than that of magnesium, but larger than that of silicon. Aluminum has a relatively high ionization energy and electronegativity, and it can form a range of chemical compounds, including aluminum oxide (Al2O3) and aluminum chloride (AlCl3).
Silicon

Silicon is a metalloid with atomic number 14. It is the second most abundant element in the Earth’s crust, and is widely used in various applications, such as electronics, solar cells, and semiconductors. Silicon has a gray, metallic appearance and a crystalline structure. Its atomic radius is smaller than that of aluminum, and it has a higher ionization energy and electronegativity than aluminum. Silicon is a semiconductor, meaning that its electrical conductivity can be controlled by adding impurities, a process known as “doping”. Silicon is also used in the production of silica, a common compound used in the manufacture of glass, ceramics, and cement.
Phosphorus

Phosphorus is a nonmetal. It is a highly reactive and essential element for life, found in many biological molecules such as DNA and ATP. In its pure form, phosphorus is a white or yellowish waxy substance that can ignite spontaneously in air. It is found in nature mainly in the form of phosphate minerals, which are used to produce fertilizers, detergents, and other products. Phosphorus has several allotropes, including white, red, and black phosphorus, each with different physical and chemical properties.
Sulfur

Sulfur is a non-metallic chemical element with the symbol S. It is an abundant, multivalent nonmetal that is found in many minerals and also in the free state as a yellow crystalline solid or powder. Sulfur is used in the production of sulfuric acid, fertilizers, and other chemicals, and is an essential element for life, found in amino acids and other biomolecules. It has many allotropes, including several forms of yellow, orange, and red sulfur, as well as amorphous and crystalline forms. Sulfur is also known for its distinctive odor, and is used in various industrial and consumer applications, such as in the production of rubber, detergents, and fungicides.
Chlorine

Chlorine is the 17th element of the periodic table and is classified as a halogen. It is a yellow-green gas at room temperature and is highly reactive due to its seven valence electrons. Chlorine is a strong oxidizing agent and can form compounds with most elements. It is widely used in the production of a range of chemicals, such as PVC, solvents, and chlorates. It is also used to disinfect water and in the production of pharmaceuticals.
Argon
Argon is the 18th element of the periodic table and is classified as a noble gas. It is a colorless, odorless, and tasteless gas at room temperature and is the third most abundant gas in the Earth’s atmosphere. Argon is inert and does not react with any other element, which makes it useful in welding and other industrial applications. It is also used in lighting and as a protective gas in the production of semiconductors. Due to its high abundance, argon is relatively cheap and widely available for use in various applications.
Properties
Melting and boiling point
The melting and boiling points of period 3 elements vary widely. Sodium and magnesium, the first two metallic elements in the period, have relatively low melting and boiling points compared to the latter elements in the period due to their weaker metallic bonds. Aluminum and silicon also have metallic bonding but have much higher melting and boiling points due to their giant covalent structures and stronger metallic bonds.
Phosphorus and sulfur, which are non-metallic elements, have even lower melting and boiling points compared to sodium and magnesium due to their weak van der Waals forces.
Chlorine, a halogen, is a gas at room temperature and pressure, with a low boiling point due to weak intermolecular forces holding its diatomic molecules together. Argon, the noble gas at the end of the period, remains a gas even at very low temperatures and high pressures, as it has a closed-shell electronic configuration and does not form chemical bonds easily.
Electronegativity
Electronegativity is a measure of an atom’s ability to attract electrons towards itself when forming a chemical bond. In period 3 elements, electronegativity generally increases from left to right across the period. This means that atoms on the right side of the period, such as chlorine, have a higher electronegativity than atoms on the left side, such as sodium.
The trend in electronegativity across the period is due to the increase in effective nuclear charge, which refers to the net positive charge experienced by an electron in an atom. As the atomic number increases across the period, the number of protons in the nucleus increases, which increases the effective nuclear charge. This increase in effective nuclear charge attracts electrons more strongly towards the nucleus, making it harder for them to be removed and increasing the electronegativity.
Metallic and nonmetallic properties
The metallic properties of period 3 elements are observed in sodium, magnesium, and aluminum. These elements are known for their ability to form cations, and their strong metallic bonds. Sodium and magnesium, for example, are highly reactive metals that can easily lose their outermost electrons to form cations. Aluminum, on the other hand, is a metal that is known for its strength and durability, and is used in a variety of industrial applications. Argon, a noble gas, is unique in that it does not form chemical bonds with other elements, and is therefore chemically inert.
The non-metallic properties of period 3 elements are observed in phosphorus, sulfur, and chlorine. These elements are known for their ability to form anions, and their weak van der Waals forces of attraction. Silicon is a metalloid that is used in a variety of electronic applications, including computer chips and solar cells. Phosphorus is an important element in biological systems and is used in fertilizers and other agricultural applications. Sulfur is a yellow, brittle, non-metallic element that is used in a variety of industrial applications, including the production of sulfuric acid. Chlorine is a highly reactive, greenish-yellow gas that is used as a disinfectant and in the production of a variety of chemicals.
Electron configuration
All period 3 elements have their valence electrons in the 3rd energy level, which means they have similar chemical and physical properties. This similarity in electronic configuration leads to similar reactivity with other elements, such as the ability to form covalent compounds with oxygen. However, there are variations in the reactivity of these elements due to their different electronegativity values.
Atomic size
As you move from left to right across period 3 elements, the atomic radius decreases. This is because the increasing nuclear charge causes the electrons to be pulled closer to the nucleus. The decrease in atomic size is most significant between sodium and magnesium, which makes it difficult to fit magnesium into the crystal structure of most sodium compounds. However, the trend in atomic size is not as clear for elements going down the group. While the atomic radius generally increases, the increase is not as pronounced as in other groups. This is due to the increasing number of electrons and energy levels, which makes the atomic radius less affected by the increasing nuclear charge.
Related
More topics
- Period 1 element
- Period 2 element
- Period 3 element
- Period 4 element
- Period 5 element
- Period 6 element
- Period 7 element
External links
- Period 3 element – Wikipedia
- Physical Properties of Period 3 Elements – Chemistry LibreTexts
- Period 3 Elements: Periodic Table, Properties, Reactions & Trends – Vaia
- atomic and physical properties of period 3 elements – Chemguide
- Periodic Table: Trends Across Period 3 Chemistry Tutorial – AUS-e-TUTE
- Trends: Melting Point and Atomic Radius Across Period 3 – Study Mind
- A Level Chemistry Revision – Period 3 Elements – chemicals.co.uk
- Trends in Period 3 Elements – ScienceAid
- Reactions of Period 3 Elements and Their Oxides – A-Level Chemistry
- Melting and boiling points across period 3 – Creative Chemistry
- Period 3 Element – Encyclopedia – Academic Accelerator
- Period 3 element – Wikiwand
- What are the trends in period 3 elements? – Quora
- About: Period 3 element – DBpedia
- How many elements in Period 3 are highly reactive? – Socratic
- Explain the trend in melting point of metals across period 3 elements – Homework.Study.com
- Why does period 3 of the periodic table contain 8 elements instead of 18? – Chemistry Stack Exchange
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
