
Protactinium (Pa) is a chemical element of the periodic table, located in the period 7, and has the atomic number 91. It is the third element in the actinide series. It is a shiny, silvery-gray metal whose name comes from the Greek word “protos”, which means first. It is highly toxic and is counted as one of the radioactive elements.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
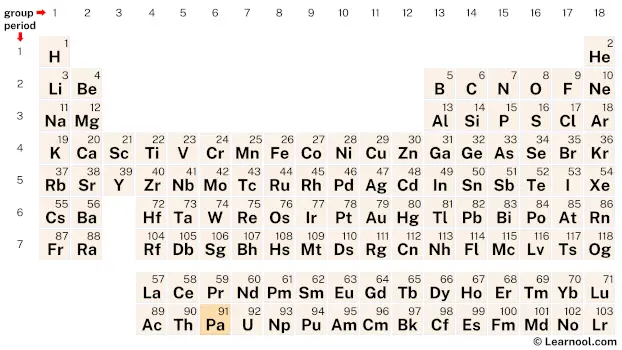
91 Pa Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – f block |
Protactinium is found in the actinide series, a group of elements located at the bottom of the periodic table. Specifically, in period 7, between thorium (Th) and uranium (U).
Element information
 |
|
 |
|
| Origin of name | Greek word “protos” (which means first) |
| Symbol | Pa |
| Atomic number (Z) | 91 |
| Atomic mass | 231.03588 u |
| Block | f-block |
| Period | 7 |
| Classification | Actinide |
| Atomic radius | 163 pm |
| Covalent radius | 200 pm |
| Melting point | 1568 ℃, 2854 ℉, 1841 K |
| Boiling point | 4027 ℃, 7280 ℉, 4300 K |
| Electron configuration | [Rn] 5f2 6d1 7s2 |
| Electrons per shell | 2, 8, 18, 32, 20, 9, 2 |
| Crystal structure | Body-centered tetragonal |
| Phase at r.t | Solid |
| Density near r.t | 15.37 g/cm3 |
| Natural occurrence | From decay |
| Oxidation state | +5 |
| Electronegativity (Pauling scale) | 1.5 |
| Protons Neutrons Electrons |
91 140 91 |
| CAS number | 7440-13-3 |
| Discovered by | Kasimir Fajans and Oswald Helmuth Göhring in 1913 |
History
Protactinium was discovered in 1913 by two scientists, Kasimir Fajans and Oswald Helmuth Göhring, while they were studying the radioactive decay of uranium. They observed an unknown radioactive substance that they identified as a new element and named it “protactinium,” meaning “precursor to actinium,” because of its position in the decay chain of uranium-235.
The discovery of protactinium was significant because it provided further evidence for the existence of isotopes, which had only recently been discovered. The properties of protactinium, such as its high radioactivity and short half-life, also made it an interesting subject of study for scientists in the early 20th century.
Protactinium remained a rare and poorly understood element for many years after its discovery, due to its scarcity and the difficulty of isolating it. It was not until the 1930s that scientists were able to produce larger quantities of protactinium, which allowed for more detailed studies of its properties and uses.
During World War Ⅱ, protactinium was used as part of the Manhattan Project to produce the first atomic bomb. It was used as a neutron source in the production of uranium-233, which was one of the fuels used in the bomb. After the war, protactinium continued to be used in nuclear research and as a source of neutrons for scientific experiments. Today, protactinium remains a valuable tool for scientists studying nuclear physics and chemistry.
Occurrence and production
Protactinium is a rare and radioactive element, so its occurrence in nature is limited. It can be found in very small quantities in minerals like pitchblende, uraninite, and monazite, and it is also produced through the decay of uranium and thorium isotopes. The main method of producing protactinium is through nuclear reactions, particularly by bombarding uranium-238 or thorium-232 with neutrons. Protactinium can also be produced through the extraction of uranium from spent nuclear fuel.
The production of protactinium is challenging because it is highly radioactive and has a relatively short half-life of about 32,000 years. The element is typically produced in small amounts as a byproduct of uranium or thorium production, and it is separated from these elements through a series of chemical and physical processes. One method involves dissolving uranium ore in acid, separating the uranium and protactinium with an organic solvent, and then chemically purifying the protactinium.
Research is ongoing to improve the production and purification methods for protactinium, particularly for use in nuclear reactors and as a precursor to medical isotopes.
Properties
Protactinium is a silvery-gray metal that is highly reactive and tarnishes quickly in air.
It is a dense metal with a density of 15.37 g/cm3 and has a melting point of 1568 ℃ and a boiling point of 4027 ℃.
Protactinium is a radioactive element with no stable isotopes, and its most stable isotope, protactinium-231, has a half-life of 32,760 years.
There are 29 known isotopes of protactinium (Pa), with atomic masses ranging from 211 to 239. However, only one of these isotopes, protactinium-231, occurs naturally and is considered to be a primordial nuclide.
Protactinium has a complex electron configuration due to the presence of the inner 5f and 6d orbitals.
Protactinium belongs to the actinide series and shares many properties with other actinides, such as uranium and thorium.
Protactinium has a high affinity for oxygen and reacts readily with most non-metals and halogens.
Its chemical properties are intermediate between those of thorium and uranium, and it is soluble in acids but insoluble in alkalis.
Applications
Protactinium has potential use as a nuclear fuel. It can be used as a nuclear reactor fuel or as a target for the production of fissile uranium-233.
Protactinium-233 is used in research as a tracer for studying the behavior of various chemical and biological systems.
Protactinium-231 has been used in dating techniques to determine the age of rocks and sediments. It has a half-life of about 32,760 years.
Protactinium-234m is used in some medical applications as a tracer for the study of bone metabolism.
Interesting facts
Protactinium is a rare and highly radioactive element, making it difficult to study and handle in large quantities.
Protactinium has no stable isotopes, meaning all of its isotopes are radioactive and decay over time.
The discovery of protactinium was initially kept secret during World WarⅡ due to its potential use in nuclear weapons.
Protactinium has a half-life of around 32,000 years, which makes it useful for dating materials up to around 350,000 years old.
The chemical behavior of protactinium is similar to that of other actinide elements, such as uranium and thorium.
Protactinium has been used in scientific research to investigate nuclear physics, radiation chemistry, and the properties of other elements.
Related
More elements
External links
- https://www.rsc.org/periodic-table/element/91/protactinium
- https://en.wikipedia.org/wiki/Protactinium
- https://www.britannica.com/science/protactinium
- https://pubchem.ncbi.nlm.nih.gov/element/Protactinium
- https://www.chemicool.com/elements/protactinium.html
- https://www.livescience.com/39728-protactinium.html
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
