
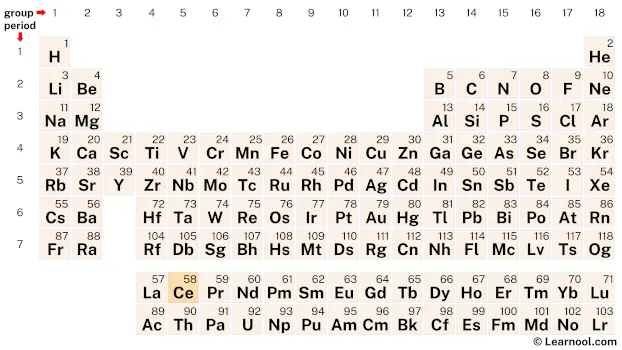
Cerium (Ce) is a chemical element of the periodic table, located in the period 6, and has the atomic number 58. It is the second element in the lanthanide series. It is a soft, ductile, silvery-white metal, which is named after the dwarf planet “Ceres”. It is the most abundant element, and is counted as one of the rare earth elements.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – f block |
Cerium is a member of the lanthanide series, a group of elements located at the bottom of the periodic table. It can be found in period 6, between lanthanum (La) and praseodymium (Pr).
Element information
 |
|
 |
|
| Origin of name | named after the dwarf planet “Ceres” |
| Symbol | Ce |
| Atomic number (Z) | 58 |
| Atomic mass | 140.116 u |
| Block | f-block |
| Period | 6 |
| Classification | Lanthanide |
| Atomic radius | 181.8 pm |
| Covalent radius | 204±9 pm |
| Melting point | 795 ℃, 1463 ℉, 1068 K |
| Boiling point | 3443 ℃, 6229 ℉, 3716 K |
| Electron configuration | [Xe] 4f1 5d1 6s2 |
| Electrons per shell | 2, 8, 18, 19, 9, 2 |
| Crystal structure | Double hexagonal close-packed (dhcp) |
| Phase at r.t | Solid |
| Density near r.t | 6.770 g/cm3 |
| Main isotopes | Cerium-136, Cerium-138, Cerium-140, Cerium-142 |
| Natural occurrence | Primordial |
| Oxidation state | +3, +4 |
| Electronegativity (Pauling scale) | 1.12 |
| Protons Neutrons Electrons |
58 82 58 |
| Learn how to find: Cerium protons neutrons electrons | |
| CAS number | 7440-45-1 |
| Discovered by | Martin Heinrich Klaproth, Jöns Jacob Berzelius, and Wilhelm Hisinger in 1803 |
History

Cerium was first discovered in 1803 by Swedish chemist Jöns Jacob Berzelius and Wilhelm Hisinger. At the same time, Martin Heinrich Klaproth, a German chemist, also independently discovered cerium in a sample of a mineral from a mine near Bodenmais, Germany. The mineral was later named cerite after the newly discovered element.
Berzelius and Hisinger obtained a brownish-red oxide of cerium from the mineral cerite and named the new element after the dwarf planet Ceres, which had been discovered two years earlier. However, Klaproth believed that the new element was a compound of tungsten and did not recognize it as a new element until 1807 when he revisited his notes and experiments.
Cerium was initially regarded as a rare and unimportant element, but its use and importance grew rapidly over time, particularly in the field of catalysis. Cerium is now used in various applications, including the production of fuel additives, glass polishing, and as a catalyst in the automotive industry.
Occurrence and production
Cerium is the most abundant of the rare earth elements, making up about 0.0046% of the Earth’s crust. It is found in a variety of minerals such as bastnasite, monazite, and xenotime. Cerium is usually obtained from these minerals through a process called solvent extraction, which involves using an organic solvent to selectively extract cerium ions from the mineral solution. The extracted cerium ions are then precipitated as cerium oxalate, which is heated to produce cerium oxide.
Another method for producing cerium is through the reduction of cerium fluoride with calcium metal. This process yields a mixture of cerium and calcium, which is then separated by a series of chemical reactions. The resulting cerium metal is typically further processed to remove any remaining impurities.
Cerium production is largely concentrated in China, which accounts for the majority of the world’s cerium supply. Other sources of cerium include the United States, Australia, Brazil, and India.
Properties
Cerium is a silvery-white metal that is malleable, ductile, and soft enough to be cut with a knife.
It has a density of 6.77 g/cm3 and a melting point of 795 ℃.
Cerium has a hexagonal close-packed crystal structure.
Cerium exhibits both +3 and +4 oxidation states, with the +3 state being more common.
Cerium is a good reducing agent, and is used in the production of iron and other metals from their ores.
Cerium can absorb large amounts of hydrogen, making it useful in hydrogen storage technologies.
Cerium is also used as a catalyst in the petroleum industry, and in the production of glass and ceramics.
It has an electronegativity of 1.12 on the Pauling scale, making it a moderately reactive element.
Applications
Catalyst
Cerium is used as a catalyst in a variety of chemical reactions, such as in the production of gasoline and other fuels. In these processes, cerium oxide helps to remove impurities from the fuel, which improves its quality and performance.
Glass polishing
Cerium oxide is used in the glass industry as a polishing agent for glass and other materials. It can be used to remove scratches and other imperfections from glass surfaces, leaving them smooth and clear.
Automotive industry
Cerium is used in the production of catalytic converters, which help to reduce emissions from automobiles. Cerium can also be used in the manufacturing of spark plugs, which helps to increase their lifespan and efficiency.
Phosphors
Cerium is used as a component in the production of phosphors, which are materials that emit light when exposed to radiation. These phosphors are used in a variety of applications, such as in television and computer screens, fluorescent lighting, and X-ray machines.
Pigments
Cerium is used in the production of pigments for a variety of applications, including paints, ceramics, and plastics. These pigments can provide a wide range of colors, and are often more stable and durable than other types of pigments.
Fuel cell technology
Cerium is used as a component in solid oxide fuel cells, which are used to generate electricity. In these fuel cells, cerium helps to improve the efficiency of the reaction, allowing for more efficient energy generation.
Nuclear energy
Cerium is used in the nuclear industry as a neutron absorber. This helps to control the rate of nuclear reactions, and can be used to prevent the buildup of excess radiation in nuclear reactors.
Interesting facts
Cerium has the highest specific heat of any element, meaning it can store more thermal energy than any other element.
Cerium is used as a doping agent in glass and optical fibers to enhance their refractive index, and it is also used in the production of camera lenses.
Cerium is the most abundant of the rare earth metals, and it is widely used in the automotive industry to reduce emissions in catalytic converters.
Cerium can ignite spontaneously in air, making it a potential fire hazard.
The color-changing properties of cerium compounds have led to their use in self-cleaning ovens and windows that change color to block sunlight.
Cerium has a unique ability to exist in two oxidation states simultaneously, making it useful in redox reactions in industrial processes.
Cerium oxide has been shown to have potential as a sunscreen due to its ability to block both UVB and UVA radiation.
Related
More elements
External links
- https://www.rsc.org/periodic-table/element/58/cerium
- https://en.wikipedia.org/wiki/Cerium
- https://www.britannica.com/science/cerium
- https://www.livescience.com/37606-cerium.html
- https://www.chemicool.com/elements/cerium.html
- https://pubchem.ncbi.nlm.nih.gov/element/Cerium
- https://study.com/academy/lesson/cerium-element-discovery-properties.html
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
