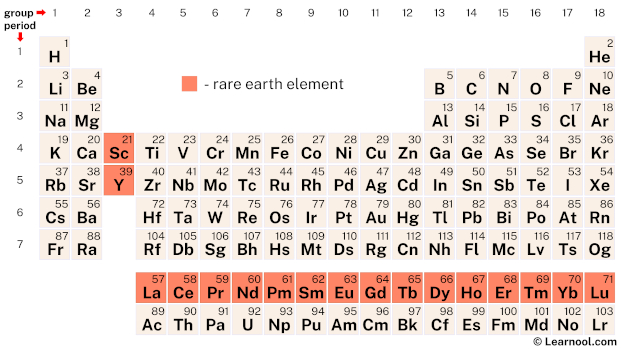
Rare-earth elements, also known as rare-earth metals or rare-earths, comprise a set of 17 chemical elements including scandium, yttrium, and the 15 lanthanides. Scandium and yttrium are considered rare-earth elements because they exhibit similar chemical properties to the lanthanides and are commonly found in the same ore deposits.
These elements find diverse applications in various fields. For example, neodymium, a rare-earth element, is crucial for the production of powerful magnets used in computer hard drives and electric motors. Cerium, another rare-earth element, is used in catalytic converters to reduce emissions in automobiles. Despite the name “rare-earth,” these elements are not actually scarce. For instance, cerium is more abundant in the Earth’s crust than lead, a common metal used in various applications, and lanthanum is more abundant than gold, a highly valued precious metal.
Rare-earth elements react differently with water. While some, like lanthanum, readily react with water to form hydrogen gas, others, such as cerium, exhibit limited reactivity. Rare-earth elements often have a silvery appearance and can tarnish when exposed to air. They possess multiple isotopes, some of which are naturally occurring, while others may have synthetic isotopes created in laboratories. Prominent scientists like Carl Gustaf Mosander and Martin Heinrich Klaproth made significant contributions to the early research and understanding of these elements.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – rare-earth element |
The periodic table encompasses a total of 17 chemical elements – scandium (Sc), yttrium (Y), and the 15 lanthanides – that fall under the category of rare-earth elements.
Related
More topics
- Transuranium element
- Superheavy element
- Rare-earth element
- Synthetic element
- Main-group element
- Inner transition metal
External links
- Rare-earth element – Wikipedia
- What are rare earth elements, and why are they important? – American Geosciences Institute
- Rare Earth Elements – Department of Energy (.gov)
- Rare Earths Statistics and Information – United States Geological Survey (.gov)
- Rare Earth Elements – Rare Element Resources
- REE – Rare Earth Elements – Metals, Minerals, Mining, Uses – Geology.com
- What are rare earths? – Lynas Rare Earths
- Rare Earth Element – an overview – ScienceDirect
- Rare Earth Elements – Massachusetts Institute of Technology
- Rare-earth element | Uses, Properties, & Facts – Britannica
- History and Future of Rare Earth Elements – Science History Institute
- Rare earth elements facts – Natural Resources Canada
- Don’t Panic about Rare Earth Elements – Scientific American
- Rare Earth Elements, Explained – Global X ETFs
- The Not-So-Rare Earth Elements: A Question of Supply and Demand – Kleinman Center of Energy Policy
- TENORM: Rare Earths Mining Wastes – United States Environmental Protection Agency (.gov)
- Geology and Mineral Resources – Rare Earth Elements – Virginia Department of Energy (.gov)
- Rare Earth Elements – Geoscience Australia
- Rare Earth Elements Supply Chain – Texas Comptroller (.gov)
- What are ‘rare earths’ used for? – BBC
- Rare Earth Elements: Where in the World Are They? – Visual Capitalist
- Rare earth elements: frequently asked questions – Wood Mackenzie
- Rare Earth Elements (Metals) – List – ThoughtCo
- Rare Earth Elements and the Race to Control Them – Boston University
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
