
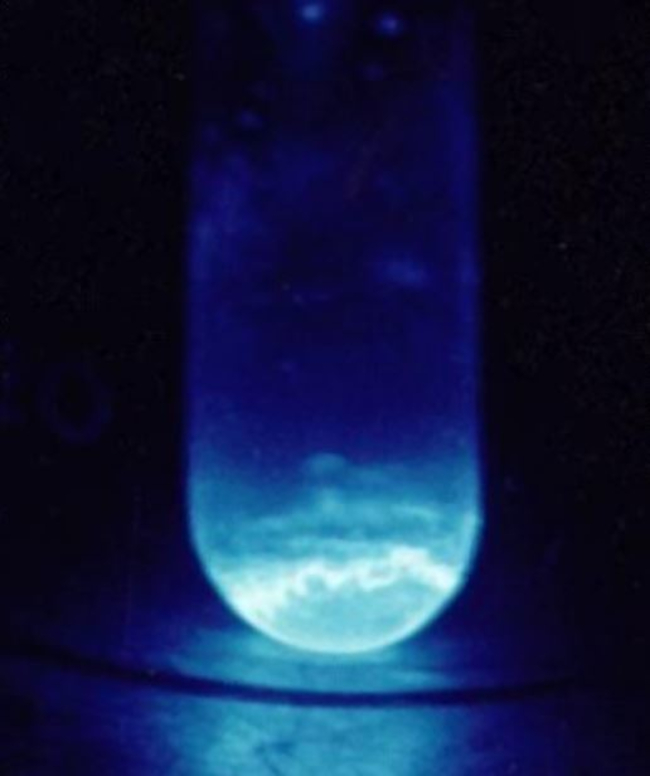
Einsteinium (Es) is a chemical element of the periodic table, located in the period 7, and has the atomic number 99. It is the eleventh element in the actinide series. It is a soft, silvery metal that glows in the dark with a blue light. It is named after the German-born theoretical physicist Albert Einstein. It is the seventh transuranium element and is counted as one of the radioactive elements.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – f block |
Einsteinium is a member of the actinide series, a group of elements located at the bottom of the periodic table. It can be found in period 7, between californium (Cf) and fermium (Fm).
Element information
 |
|
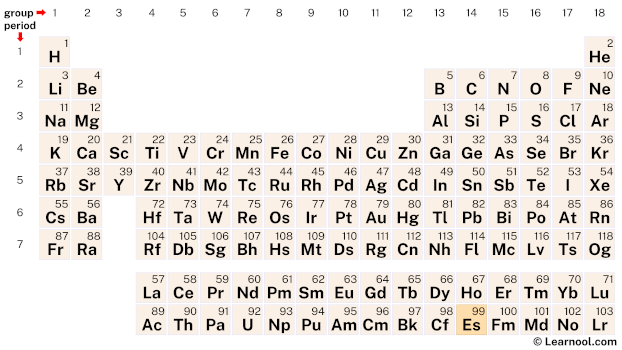 |
|
| Origin of name | named after German-born theoretical physicist Albert Einstein |
| Symbol | Es |
| Atomic number (Z) | 99 |
| Atomic mass | (252) |
| Block | f-block |
| Period | 7 |
| Classification | Actinide |
| Melting point | 860 ℃, 1580 ℉, 1133 K |
| Boiling point | 996 ℃, 1825 ℉, 1269 K (estimated) |
| Electron configuration | [Rn] 5f11 7s2 |
| Learn how to write: Einsteinium electron configuration | |
| Electrons per shell | 2, 8, 18, 32, 29, 8, 2 |
| Learn how to draw: Einsteinium Bohr model | |
| Crystal structure | Face-centered cubic (fcc) |
| Phase at r.t | Solid |
| Density near r.t | 8.84 g/cm3 |
| Natural occurrence | Synthetic |
| Oxidation state | +3 |
| Electronegativity (Pauling scale) | 1.3 |
| Protons Neutrons Electrons |
99 153 99 |
| CAS number | 7429-92-7 |
| Discovered at | Lawrence Berkeley National Laboratory in 1952 |
History

Einsteinium was first discovered in 1952 by a team of scientists led by Albert Ghiorso at the University of California, Berkeley. It was named after Albert Einstein, the renowned physicist who developed the theory of relativity.
Einsteinium does not occur naturally in the environment, and is instead produced in nuclear reactors through the bombardment of uranium atoms with neutrons. Due to its synthetic nature, it is considered a rare and highly valuable element in scientific research.
The discovery of einsteinium was a significant achievement in the field of nuclear chemistry, as it represented the first time that a new element had been synthesized in quantities large enough to enable further study. Einsteinium’s unique properties have since been studied extensively in fields such as materials science, nuclear medicine, and nuclear energy.
Today, einsteinium is primarily used for research purposes, particularly in the fields of nuclear chemistry and nuclear physics. Its radioactive properties make it an ideal subject for studying the behavior of heavy elements and the mechanisms of nuclear reactions. While it has no practical applications outside of the laboratory, the continued study of einsteinium and other synthetic elements is crucial for advancing our understanding of the fundamental properties of matter.
Occurrence and production
Einsteinium is an artificially produced element, which means it is not found naturally in the Earth’s crust. It was first synthesized in 1952 by a team of scientists led by Glenn T. Seaborg at the University of California, Berkeley. The element was named after Albert Einstein to honor his contributions to theoretical physics.
Einsteinium is produced through nuclear reactions involving heavy isotopes of other elements, such as uranium and plutonium. The most common method of producing einsteinium is by bombarding uranium-238 with neutrons in a nuclear reactor, which produces uranium-239. The uranium-239 then undergoes a series of beta decays to become plutonium-239, which can be further irradiated with neutrons to produce einsteinium-253.
The production of einsteinium is extremely difficult and expensive due to its short half-life of only 20.5 days. This means that any samples of the element must be produced quickly and then rapidly transported to their destination for analysis or use. Currently, only a few milligrams of einsteinium have ever been produced, making it one of the rarest and most expensive elements on the planet.
Properties
Physical properties
Einsteinium is a silvery-white metal that is radioactive and highly reactive. It has a melting point of 860 ℃ and a boiling point of approximately 996 ℃.
Chemical properties
Einsteinium is highly reactive and is capable of forming compounds with a wide range of elements. It is a strong oxidizing agent and reacts readily with oxygen, halogens, and other elements.
Atomic properties
Einsteinium has an atomic number of 99 and is located in the actinide series of the periodic table. It has 99 protons and 99 electrons, with its most stable isotope being 252Es.
Radioactive properties
Einsteinium is highly radioactive and has a very short half-life of only a few weeks. It emits alpha, beta, and gamma rays and undergoes a process of spontaneous fission.
Chemical behavior
Einsteinium behaves similarly to other actinides, such as uranium and plutonium, due to their similar electronic configurations.
Magnetic properties
Einsteinium has been found to have magnetic moments that are aligned in the same direction as those of its neighboring elements in the periodic table.
Optical properties
Einsteinium has no notable optical properties, but it has been studied for its potential use in lasers due to its high radioactivity.
Applications
Nuclear research
As an artificial element, einsteinium has been used extensively in nuclear research, particularly for studying the behavior of radioactive isotopes and the properties of nuclear particles.
Neutron sources
Einsteinium is one of the few elements that can be used as a neutron source, emitting high-energy neutrons when bombarded with alpha particles.
Medical applications
Despite being highly radioactive and dangerous to handle, einsteinium has been investigated for potential medical applications such as targeted radiotherapy and imaging.
Industrial radiography
Einsteinium can be used as a gamma ray source for industrial radiography, allowing for non-destructive testing of materials and structures.
Basic scientific research
Like other transuranic elements, einsteinium is studied for its fundamental properties and to further our understanding of the nature of matter.
Note: It should be noted that due to the extreme rarity and high cost of einsteinium, many of these applications are only theoretical or experimental in nature, and may not be practical on a larger scale.
Interesting facts
Einsteinium was first discovered as part of the debris from the first thermonuclear bomb test in 1952.
The element is named after physicist Albert Einstein.
Einsteinium is the seventh transuranic element.
The most stable isotope of einsteinium is einsteinium-252, which has a half-life of 471.7 days.
Einsteinium is a soft, silvery-white, radioactive metal that is highly reactive with air and water.
It has no known biological role, but it has been used in medical research to study the behavior of cells and DNA.
Einsteinium has been used as a target material for nuclear reactions to produce other heavy elements, such as mendelevium and nobelium.
Einsteinium has only been produced in small amounts, and there are no known commercial applications for it.
Einsteinium is not found in nature and can only be produced by bombarding lighter elements with neutrons in a nuclear reactor.
Related
More elements
External links
- https://www.britannica.com/science/einsteinium
- https://www.rsc.org/periodic-table/element/99/einsteinium
- https://en.wikipedia.org/wiki/Einsteinium
- https://www.chemicool.com/elements/einsteinium.html
- https://www.livescience.com/40307-einsteinium.html
- https://pubchem.ncbi.nlm.nih.gov/element/Einsteinium
- https://study.com/academy/lesson/einsteinium-element-discovery-name-properties.html
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
