
Rubidium (Rb) is a chemical element of the periodic table, located in group 1 and period 5, and has the atomic number 37. It is a very soft, silvery-white alkali metal whose name comes from the Latin word “rubidus” (which means the deepest red). It was discovered in 1861 by Robert Bunsen and Gustav Kirchhoff, who observed its spectral lines while studying the emission spectra of various elements.
Rubidium and cesium are utilized interchangeably or simultaneously in many applications due to their similar physical properties and atomic radii. However, cesium has a higher electropositivity than rubidium, making it a more suitable material for particular applications. One to two metric tons of rubidium is sold in the United States each year, which is a very small market.[1] Its primary application is in specialty glasses used in fiber optic telecommunication systems.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – s block |
Rubidium is an s-block element, situated in the first column of the periodic table, below potassium (K). It has the atomic number 37 and is denoted by the symbol Rb.
Element information
 |
|
 |
|
| Origin of name | Latin word “rubidus” (which means the deepest red) |
| Symbol | Rb |
| Atomic number (Z) | 37 |
| Atomic mass | 85.4678 u |
| Block | s-block |
| Group | 1 |
| Period | 5 |
| Classification | Alkali metal |
| Atomic radius | 248 pm |
| Covalent radius | 220±9 pm |
| Van der Waals radius | 303 pm |
| Melting point | 39.30 ℃, 102.74 ℉, 312.45 K |
| Boiling point | 688 ℃, 1270 ℉, 961 K |
| Electron configuration | [Kr] 5s1 |
| Electrons per shell | 2, 8, 18, 8, 1 |
| Crystal structure | Body-centered cubic (bcc) |
| Phase at r.t | Solid |
| Density near r.t | 1.532 g/cm3 |
| Main isotopes | Rubidium-85 |
| Natural occurrence | Primordial |
| Oxidation state | +1 |
| Electronegativity (Pauling scale) | 0.82 |
| Protons Neutrons Electrons |
37 48 37 |
| Valence electrons | 1 |
| Learn how to find: Rubidium valence electrons | |
| CAS number | 7440-17-7 |
| Discovered by | Robert Bunsen and Gustav Kirchhoff in 1861 |
History
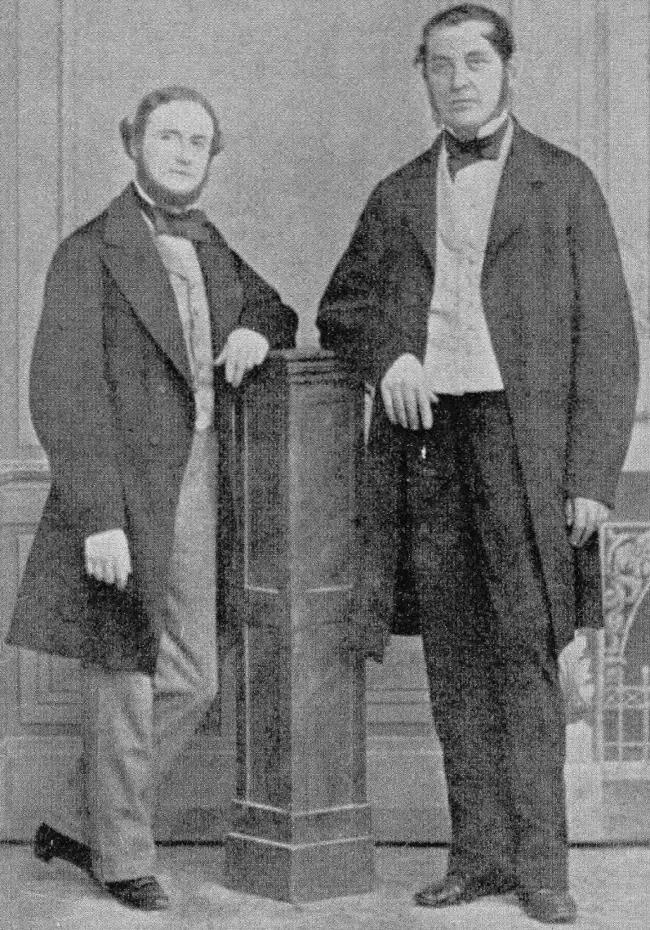
Rubidium was discovered in 1861 at Heidelberg, Germany, by Robert Bunsen and Gustav Kirchhoff while examining the mineral lepidolite using flame spectroscopy. Its name is derived from the color of the two prominent lines in the red portion of the element’s spectrum.[2]
Rubidium was the second element found via spectroscopy, immediately after cesium, just one year after Bunsen and Kirchhoff invented the spectroscope. It had almost no industrial uses until the 1920s, when small amounts were utilized in photoelectric cells. Since then, much of the rubidium consumed has been utilized in various types of research.
Occurrence and production
In the Earth’s crust, natural rubidium makes up roughly 0.01 percent. It is more abundant than some popular metals like copper, lead, and zinc, all of which are mined at quantities measured in the millions of metric tonnes per year, as compared to rubidium’s possible global mining quantity of perhaps 2 to 4 tonnes per year. Rubidium can be found naturally in minerals such as leucite, pollucite, carnallite, and zinnwaldite. Some potassium rocks and brines also contain considerable amounts of the element.
Rubidium is difficult to produce in pure form because it naturally occurs alongside cesium and other alkali metals. Hence, pure rubidium must be extracted from its ores lepidolite and pollucite and then separated from potassium and cesium through a series of chemical processes. Methods like fractional crystallization, chlorostannate process, and ferrocyanide process have been used to extract rubidium.
Properties
Rubidium interacts violently with water, just like other alkali metals do. Additionally, it has been noted that rubidium can spontaneously burn in the air.
The metal quickly reacts with water and produces a colorless solution of rubidium hydroxide RbOH and hydrogen gas H2. It produces rubidium monoxide Rb2O when exposed to air and superoxide RbO2 when exposed to too much oxygen.

Rubidium, like potassium, exhibits a very similar purple color in the flame test.
Naturally occurring rubidium has two isotopes with atomic weights of 85 and 87, which occur in amounts around 72.2% and 27.8% by weight. 87Rb has a half-life of 48.8 billion years and is radioactive.
Other rubidium isotopes, which are all very radioactive and have few uses, have been synthesized artificially.
Applications
In vacuum tubes, rubidium is utilized as a getter, a substance that mixes with and extracts trace gasses from vacuum tubes.
Rubidium is utilized in fiber optic telecommunication systems, specialty glasses, night vision devices, and other applications.
It is used in electronic device manufacturing and as a frequency reference in atomic clocks.
Additionally, it is utilized in the rubidium-strontium dating method, a radiometric dating technique, which is employed by scientists to determine the age of rocks and minerals. The decay of radioactive 87Rb and 86Sr has been widely used to determine the age of rocks and minerals.

Rubidium compounds are sometimes employed in fireworks to give them a purple tint.
For positron emission tomography (PET) scans, rubidium-82 is employed.
In terms of mood, anti-conservative beliefs, work, occupational interests, and psychomotor slowness, rubidium chloride showed a marked and quick anti-depressive action.[3]
Rubidium can be quickly ionized, making it a potential propellant for ion engines on spacecraft.
Interesting facts
Rubidium has a density higher than water, and so it does not float on water.
Rubidium was discovered using a flame emission spectroscopy method.
It is the second most electropositive element among stable alkali metals.
Rubidium is the twenty-second most abundant element on earth.
Rubidium melts just above the body temperature, at 39.30 ℃.
Rubidium ignites in the air and reacts violently with water, so it is kept in mineral oil.
Related
More elements
References
1. Mineral Commodity Profiles – Rubidium
2. C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – RUBIDIUM
3. Rubidium chloride in the treatment of major depression
External links
- https://www.rsc.org/periodic-table/element/37/rubidium
- https://pubchem.ncbi.nlm.nih.gov/element/Rubidium
- https://www.britannica.com/science/rubidium
- https://en.wikipedia.org/wiki/Rubidium
- https://www.livescience.com/34519-rubidium.html
- https://www.chemicool.com/elements/rubidium.html
- https://www.radiochemistry.org/periodictable/elements/37.html
- https://www.webelements.com/rubidium/
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
