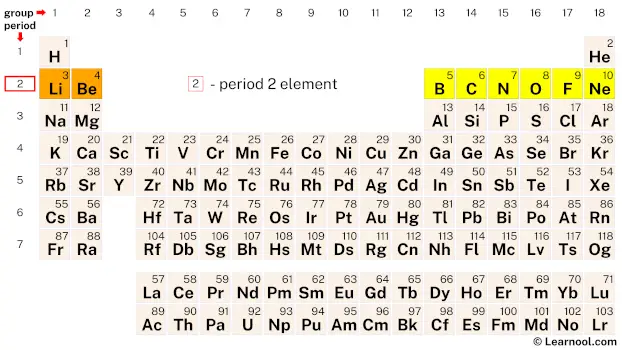
Period 2 elements are the chemical elements found in the second row or period of the periodic table. This row contains eight elements, starting from lithium (Li) and ending with neon (Ne). These elements have two electron shells, with the first shell containing two electrons and the second shell containing up to eight electrons. While most period 2 elements follow the octet rule, there are a few exceptions such as lithium and beryllium, which follow the duet rule, and boron is electron deficient and can only accommodate six electrons in its valence shell.
Period 2 elements exhibit a wide range of physical and chemical properties, which are determined by their atomic structures and electron configurations. These elements are notable for their high reactivity and ability to form a wide range of chemical compounds. For example, boron and carbon are known for their ability to form strong covalent bonds, while lithium and beryllium are highly reactive metals that readily form ionic compounds. These properties are not only important for basic scientific research but also have practical applications in fields such as materials science, electronics, and energy storage.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| 2 | – period 2 element |
Period 2 is the second row of elements on the periodic table. It contains eight elements, namely lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon.
Element information
Lithium

Lithium is a soft, silvery-white metal that is highly reactive and flammable. It is the lightest metal and the least dense solid element, with a density only about half that of water. Lithium is an important element for a number of industrial and technological applications, including the production of batteries, ceramics, glass, and aluminum. It is also used in medicine, as lithium carbonate is a common treatment for bipolar disorder.
Beryllium

Beryllium is a hard, grayish-white metal that is brittle at room temperature but becomes ductile and malleable when heated. It is a very light and strong metal with excellent thermal conductivity, and is used in a variety of aerospace and defense applications, as well as in X-ray equipment and nuclear reactors. However, beryllium is also highly toxic and can cause a serious lung disease known as berylliosis, so it must be handled with care.
Boron

Boron is a metalloid element that is essential for the growth and development of plants, and is also used in a variety of industrial applications. It is a hard and brittle material with a high melting point, and can be found in the form of borax or boric acid. Boron compounds are used in the production of glass, ceramics, fertilizers, and semiconductors, as well as in nuclear reactors and other high-tech applications.
Carbon

Carbon is a non-metallic element that is essential to life and the basis of all organic compounds. It is the fourth most abundant element in the universe and can be found in a variety of forms, including diamond, graphite, and amorphous carbon. Carbon has a wide range of industrial and technological applications, including the production of steel, plastics, and carbon fibers, as well as in energy storage and conversion devices such as batteries and fuel cells.
Nitrogen

Nitrogen is a non-metallic element that makes up about 78% of the Earth’s atmosphere. It is a colorless, odorless gas that is essential for the growth and development of living organisms, and is also used in a variety of industrial applications. Nitrogen is used in the production of fertilizers, explosives, and other chemicals, as well as in cryogenics and other high-tech applications.
Oxygen

Oxygen is a non-metallic element that is essential for the survival of most living organisms. It is a colorless, odorless gas that makes up about 21% of the Earth’s atmosphere, and is also found in a variety of other forms, including water and various oxides. Oxygen is used in a wide range of industrial and medical applications, including the production of steel, glass, and chemicals, as well as in respiratory therapy and other medical treatments.
Fluorine

Fluorine is a highly reactive nonmetal element that is the most electronegative element in the periodic table. It is a pale yellow gas at room temperature, and is highly toxic and corrosive. Fluorine is used in a variety of industrial and technological applications, including the production of uranium, plastics, and various chemicals. It is also used in dental treatments, as fluorine compounds are added to toothpaste and water supplies to prevent tooth decay.
Neon

Neon is a noble gas that is colorless, odorless, and nearly inert under normal conditions. It is the second lightest noble gas and the second least reactive element in the periodic table, after helium. Neon is used in a variety of lighting applications, including neon signs and fluorescent lamps. It is also used in cryogenics and as a coolant in gas-cooled nuclear reactors. Neon has no known biological role and is considered to be non-toxic, making it a safe choice for various industrial and commercial applications.
Physical properties
Period 2 elements share some common physical properties, such as small atomic radii, high ionization energies, and low melting and boiling points. This is due to the fact that the atomic radii of these elements decrease from left to right across the period. The ionization energies of period 2 elements increase from left to right across the period, which means that it becomes increasingly difficult to remove an electron as you move from left to right across the period. The electronegativity of these elements also generally increases from left to right, with fluorine being the most electronegative element in the period.
Related
More topics
- Period 1 element
- Period 2 element
- Period 3 element
- Period 4 element
- Period 5 element
- Period 6 element
- Period 7 element
External links
- Period 2 element – Wikipedia
- 3.5: The Second Period of the Periodic Table – Chemistry LibreTexts
- Trends Across Period 2 Chemistry Tutorial – AUS-e-TUTE
- What are the elements in Period 2 that are metals? – Socratic
- About: Period 2 element – DBpedia
- Period 2 Element – Academic Accelerator
- Octet rule violation in Period 2 elements – Chemistry Stack Exchange
- How to Write the Electron Configuration for Elements in the 2nd Period of the Periodic Table – Study.com
- Period 2 elements Flashcards – Quizlet
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
