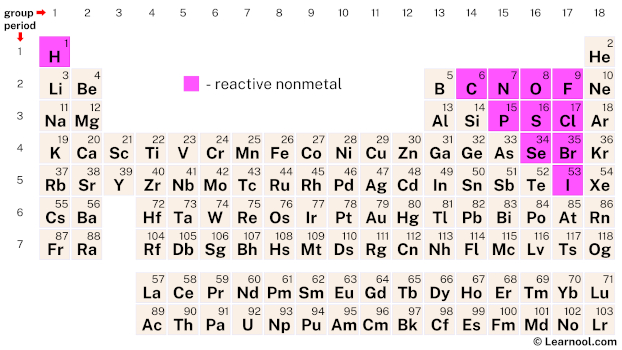
Reactive nonmetals are a group of chemical elements that are located in the upper right-hand corner of the periodic table. This group includes 11 elements, namely hydrogen (H), carbon (C), nitrogen (N), oxygen (O), fluorine (F), phosphorus (P), sulfur (S), chlorine (Cl), selenium (Se), bromine (Br), and iodine (I). These elements are characterized by their inability to conduct heat and electricity and low melting and boiling points.
Reactive nonmetals exhibit properties that are markedly different from metals. They tend to be brittle and are typically found as gases or soft solids at room temperature. One well-known example of a reactive nonmetal is fluorine, which was discovered by French chemist Henri Moissan in 1886. Fluorine is the most reactive nonmetal and can react with almost all other elements to form compounds.
Reactive nonmetals have many important applications in various fields, as well as interesting properties. For example, oxygen is essential for respiration and combustion, while nitrogen is a key component of fertilizers and is used in the production of ammonia. Carbon is the basis of all known life on Earth and is also used in various industrial processes. Reactive nonmetals also have important uses in electronics, such as in the production of semiconductors.
Some reactive nonmetals have unique and notable properties. Hydrogen, for example, is the lightest and most abundant element in the universe, and it is commonly used as fuel for rockets and as a reactant in chemical reactions. Sulfur is a nonmetal with a distinct odor and is used in the production of sulfuric acid, fertilizers, and rubber products. Iodine is a purple-black solid that sublimes into a violet gas and is commonly used as a disinfectant, a catalyst, and in the production of pharmaceuticals.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – reactive nonmetal |
Reactive nonmetals on the periodic table include 11 elements – hydrogen, carbon, nitrogen, oxygen, fluorine, phosphorus, sulfur, chlorine, selenium, bromine, and iodine.
History
Reactive nonmetals have been known since ancient times, with some of the elements, such as carbon and sulfur, being used by humans in various applications. However, it wasn’t until the late 18th and early 19th centuries that several of the reactive nonmetals were discovered and identified as distinct elements.
Hydrogen, the lightest and most abundant element in the universe, was discovered in 1766 by English chemist Henry Cavendish. Carbon, another important reactive nonmetal, has been known since ancient times, with its name derived from the Latin word “carbo,” meaning charcoal. Nitrogen was discovered as a distinct element in 1772 by Scottish physician and chemist Daniel Rutherford.
Oxygen, one of the most important reactive nonmetals due to its role in respiration and combustion, was discovered in the late 18th century by several scientists independently, including Swedish chemist Carl Wilhelm Scheele and English chemist Joseph Priestley. Fluorine, the most reactive nonmetal, was first isolated in 1886 by French chemist Henri Moissan, who used electrolysis to extract it from a mixture of hydrofluoric acid and potassium fluoride.
Phosphorus, another important reactive nonmetal, was discovered in 1669 by German alchemist Hennig Brand. Selenium, a nonmetal with important applications in electronics, was discovered in 1817 by Swedish chemist Jöns Jacob Berzelius. Chlorine, a greenish-yellow gas with a pungent odor, was discovered in 1774 by Swedish chemist Carl Wilhelm Scheele, while sulfur was known to ancient civilizations and was referred to as brimstone.
Bromine, a reddish-brown liquid that evaporates easily to form a brownish gas, was discovered in 1825-1826 by French chemist Antoine-Jérôme Balard. Its name is derived from the Greek word “bromos,” which means stench, due to its strong and unpleasant odor. Iodine, a purple-black solid that sublimes into a violet gas, was discovered in 1811 by French chemist Bernard Courtois. Its name is derived from the Greek word “iodes,” which means violet-colored.
Occurrence
Reactive nonmetals are widely distributed in the Earth’s crust and atmosphere, and they can be found in various forms such as gases, liquids, and solids. Of the reactive nonmetals, nitrogen is the most abundant in the Earth’s atmosphere, making up about 78% of the air we breathe. Oxygen is the second most abundant reactive nonmetal, comprising about 21% of the atmosphere.
Hydrogen, the lightest element, is the most abundant element in the universe and is found in a variety of compounds, including water and hydrocarbons. Carbon occurs in the Earth’s crust in the form of minerals such as graphite and diamond, as well as in organic compounds.
Phosphorus occurs in the Earth’s crust in the form of phosphate minerals and is an essential element for life. Sulfur is found in its elemental form, as well as in sulfide and sulfate minerals. Fluorine is the most electronegative element and is not found in its elemental form in nature, but rather as fluoride compounds in minerals such as fluorite, cryolite, and apatite.
Production
Reactive nonmetals are produced using a variety of methods, depending on the element and its properties. Hydrogen is primarily produced through the steam reforming of natural gas, while carbon is produced through the combustion of fossil fuels or through the reduction of carbon dioxide. Nitrogen is produced through the fractional distillation of liquid air, while oxygen is produced through the fractional distillation of air or through the electrolysis of water.
Fluorine can be produced through the electrolysis of molten fluorides, such as potassium fluoride and hydrogen fluoride, while phosphorus is produced through the reduction of phosphate rock with coke in an electric furnace. Sulfur is primarily produced through the Frasch process, which involves pumping hot water into underground sulfur deposits to melt the sulfur, which is then pumped to the surface.
Chlorine is produced through the electrolysis of brine, while selenium is commonly obtained as a byproduct of copper refining and coal combustion processes. Bromine is primarily produced through the treatment of seawater with chlorine, while iodine is primarily produced through the extraction of iodine-containing brines or through the processing of caliche ore.
Properties
Physical properties
Appearance
Reactive nonmetals are typically brittle solids, gases or soft solids at room temperature. For example, sulfur is a yellow, crystalline solid while chlorine is a greenish-yellow gas.
Melting and boiling points
Reactive nonmetals have low melting and boiling points compared to metals. For instance, nitrogen has a boiling point of -195.79 ℃, while oxygen has a melting point of -218.79 ℃.
Density
Reactive nonmetals have low densities compared to metals.
Chemical properties
Reactivity
Reactive nonmetals exhibit high reactivity and have a tendency to readily form compounds with various other elements. For instance, among all nonmetals, fluorine holds the position of being the most reactive as it can form compounds with almost all elements.
Electronegativity
Reactive nonmetals have high electronegativities, which means they tend to attract electrons towards themselves in chemical reactions. For example, oxygen has a high electronegativity and tends to form covalent bonds with other elements.
Other properties
Electrical conductivity
Reactive nonmetals are poor conductors of electricity, unlike metals.
Toxicity
Some reactive nonmetals, such as chlorine and fluorine, can be toxic in certain forms.
Isotopes
Some reactive nonmetals, such as carbon and sulfur, have several isotopes with different numbers of neutrons.
Applications
Industrial
In the industrial sector, hydrogen is used for various purposes such as in the production of ammonia, methanol, and petroleum refining. Carbon, on the other hand, is used in the manufacturing of steel, electrodes, and graphite lubricants. Nitrogen is utilized in the production of fertilizers, ammonia, and explosives, while oxygen finds its applications in the production of steel and other metals, as well as for medical and respiratory purposes.
Fluorine is used in the production of refrigerants, plastics, and pharmaceuticals, while phosphorus is used in the production of detergents and pesticides. Sulfur is employed in the production of sulfuric acid and rubber vulcanization, and chlorine is used in the production of various chemicals, including plastics, solvents, and pharmaceuticals.
Selenium is used in the manufacturing of glass and electronic components, while bromine finds its application in the production of flame retardants and water disinfectants. Finally, iodine is used in the production of pharmaceuticals and x-ray contrast agents.
Biological
Carbon is a vital component of organic molecules in living organisms, including carbohydrates, proteins, and DNA. Nitrogen is used by plants to produce proteins and nucleic acids, which are necessary for growth and reproduction. Oxygen is required for cellular respiration, the process by which organisms convert food into energy. Phosphorus is an essential element for DNA and RNA, and it also plays a crucial role in energy transfer in cells. Sulfur is used in the formation of disulfide bonds in proteins and for energy metabolism.
Chlorine is an important component of sodium chloride (table salt) which is essential for many biological processes, including maintaining proper fluid balance and transmitting nerve impulses. Iodine is another important element in living organisms, as it is necessary for the production of thyroid hormones. These hormones play a critical role in regulating metabolism, growth, and development. Iodine deficiency can lead to a range of health problems, including goiter, cretinism, and developmental delays in children.
Environmental
Reactive nonmetals play an important role in environmental applications. Hydrogen, for instance, is used as a fuel for hydrogen fuel cell vehicles, which is a cleaner alternative to traditional gasoline vehicles. Carbon is used in carbon capture and storage technologies to reduce greenhouse gas emissions and mitigate the impact of climate change. Nitrogen is a vital nutrient for plants and is used as a fertilizer to enhance crop growth, while oxygen plays a crucial role in the survival of aquatic organisms, such as fish, in water bodies.
Phosphorus is another essential element for plant growth, but it can also cause eutrophication when it enters water bodies in excess. This phenomenon results in excessive plant growth, oxygen depletion, and fish and other aquatic organisms’ death. Sulfur, on the other hand, is used in desulfurization processes to reduce sulfur dioxide emissions from industrial processes and power plants, which can cause acid rain and other environmental issues. Chlorine is commonly used to disinfect water and wastewater, and to treat drinking water, while bromine is used in water treatment as a disinfectant and to control algae growth.
Interesting facts
Hydrogen is the lightest and most abundant element in the universe and is used in the production of ammonia, methanol, and as a fuel for fuel cells.
Carbon has the highest sublimation point of all the elements, at 3642 ℃.
Nitrogen makes up about 78% of the Earth’s atmosphere and is a key component of amino acids, which are the building blocks of proteins.
Oxygen makes up about 21% of the Earth’s atmosphere, making it the most abundant reactive nonmetal on the planet.
Fluorine is the most electronegative element on the periodic table, meaning it has the highest tendency to attract electrons towards itself.
Phosphorus is the 11th most abundant element in the Earth’s crust and is used in the production of matches, fertilizers, and detergents.
Sulfur has been used for thousands of years in medicine and is still used today in the production of sulfuric acid, rubber, and fertilizers.
Chlorine was first used as a weapon in World War Ⅰ and is now widely used in the production of plastics, solvents, and paper products.
Selenium is used in the glass industry to decolorize glass and remove impurities, and it is also used in the production of pigments, rubber, and electronic components. It has semiconducting properties, which make it useful in electronics and solar cell production.
Bromine is the only liquid reactive nonmetal and is used in flame retardants, water disinfectants, and pharmaceuticals.
Iodine was discovered in 1811 by Bernard Courtois and is essential for the production of thyroid hormones, which regulate metabolism in the body.
Related
More topics
- Block (periodic table)
- Group (periodic table)
- Period (periodic table)
- Metal
- Alkali metal
- Alkaline earth metal
- Lanthanide
- Actinide
- Transition metal
- Post-transition metal
- Metalloid
- Nonmetal
- Reactive nonmetal
- Noble gas
External links
- Category:Reactive nonmetals – Wikipedia
- Nonmetals – CK-12
- What nonmetals are most chemically reactive? – Socratic
- What is the most reactive nonmetal? – Homework.Study.com
- Which nonmetals are among the most reactive? – Quora
- Characteristics of Nonmetals – Chemistry LibreTexts
- Non-Metals – Diamond Light Source
- Most reactive group of nonmetals? – Answers
- Which Is The Most Reactive Element In The Periodic Table? – Science ABC
- Reactive Nonmetals Flashcards – Quizlet
- Reactive Nonmetals | Science – ShowMe
- What Is the Most Reactive Metal? Most Reactive Element? – Science Notes and Projects
- Category:Reactive nonmetals – Wikiwand
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.


