
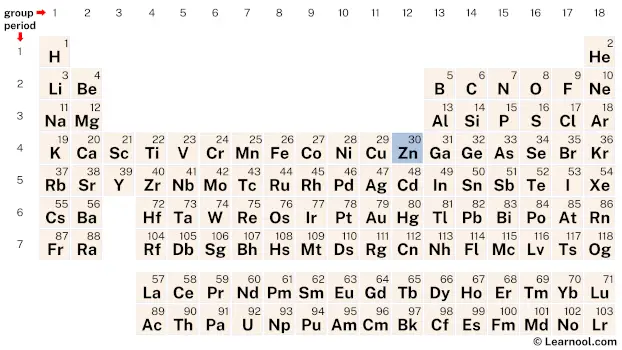
Zinc (Zn) is a chemical element of the periodic table, located in the group 12 and the period 4, and is having the atomic number 30. It is a slightly brittle, silvery-white post transition metal, whose name comes from the German word “zinke”, which means prong or tooth. It is the 24th most abundant element on earth.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – d block |
Zinc is a d-block element, found in the twelfth column and the fourth row of the periodic table. It has the atomic number 30 and is denoted by the symbol Zn.
Element information
 |
|
 |
|
| Origin of name | German word “zinke” (which means prong or tooth) |
| Symbol | Zn |
| Atomic number (Z) | 30 |
| Atomic mass | 65.38 u |
| Block | d-block |
| Group | 12 |
| Period | 4 |
| Classification | Post-transition metal |
| Atomic radius | 134 pm |
| Covalent radius | 122±4 pm |
| Van der Waals radius | 139 pm |
| Melting point | 419.53 ℃, 787.15 ℉, 692.68 K |
| Boiling point | 907 ℃, 1665 ℉, 1180 K |
| Electron configuration | [Ar] 3d10 4s2 |
| Electrons per shell | 2, 8, 18, 2 |
| Crystal structure | Hexagonal close-packed (hcp) |
| Phase at r.t | Solid |
| Density near r.t | 7.14 g/cm3 |
| Main isotopes | Zinc-64, Zinc-66, Zinc-67, Zinc-68, Zinc-70 |
| Natural occurrence | Primordial |
| Oxidation state | +2 |
| Electronegativity (Pauling scale) | 1.65 |
| Protons Neutrons Electrons |
30 35 30 |
| Learn how to find: Zinc protons neutrons electrons | |
| CAS number | 7440-66-6 |
| Discovered by | Indian metallurgists before 1000 BCE |
History
Zinc is a versatile chemical element that has a rich history dating back to ancient times. Although the Greeks and Romans were familiar with the metal, it was not until the 16th century that it was recognized as a distinct element. It wasn’t until the 17th century that a method for extracting zinc from its ores was discovered by German metallurgist Andreas Marggraf. This discovery led to the commercial production of zinc in the 18th century, primarily in Europe.
During the 19th century, the primary use of zinc was for galvanizing iron and steel. Galvanization involves coating iron or steel with a layer of zinc to protect it from corrosion. The process of galvanization played an important role in the development of the modern industrial economy, as it allowed for the widespread use of iron and steel in construction and other applications.
In the early 20th century, zinc became an important component in the production of brass alloys. Brass is a mixture of copper and zinc that is prized for its strength, durability, and attractive appearance. Today, zinc has a number of important applications in modern technology. It is used as a component in batteries, including alkaline and zinc-carbon batteries. Zinc oxide is used in a variety of products, including paints, rubber products, and pharmaceuticals.
Zinc is also an essential nutrient for human health, playing a vital role in a number of bodily functions. It is important for the immune system, wound healing, and the metabolism of proteins, carbohydrates, and fats. Zinc deficiency can lead to a range of health problems, including impaired growth and development, poor wound healing, and increased susceptibility to infection.
Occurrence and production
Zinc is a relatively abundant element on earth, and it is found in various minerals, including sphalerite (ZnS), smithsonite (ZnCO3), hemimorphite [Zn4Si2O7(OH)2 · H2O], and franklinite (ZnFe2O4). The most common source of zinc is the mineral sphalerite, which is typically found in association with other sulfide minerals in zinc-lead ore deposits.
Zinc is mainly mined in three countries: China, Peru, and Australia. China is the largest producer of zinc, accounting for around 40% of the world’s total zinc production, with most of the zinc mines located in the provinces of Shaanxi, Inner Mongolia, Hunan, and Yunnan. Peru is the second-largest producer of zinc, with its mines located in the Andes Mountains, particularly in the regions of Ancash, Junín, Pasco, and Lima. Australia is the third-largest producer of zinc, with its mines located in the Mount Isa region of Queensland and the Northern Territory. Other countries with significant zinc deposits include the United States, Mexico, Canada, Kazakhstan, and India.
Zinc is primarily produced through the process of roasting and leaching zinc sulfide concentrates, which are then electrowon to produce the metal. In the roasting process, the concentrates are roasted in air to produce zinc oxide, which is then mixed with coke and heated in a furnace to produce zinc vapor. The vapor is then oxidized to form zinc oxide, which is then reduced with carbon to produce zinc metal.
Another method of producing zinc is through the electrolysis of zinc sulfate solution, which is produced by dissolving roasted zinc sulfide concentrates in sulfuric acid. In this process, zinc sulfate solution is electrolyzed, and the resulting zinc metal is deposited on cathodes. This process is known as electrowinning and is commonly used in the production of zinc in the United States.
In addition to the above methods, zinc can also be produced by recycling zinc-containing materials, such as galvanized steel scrap, brass scrap, and zinc die-casting alloys. The recycling process involves melting the materials in a furnace and separating the zinc from other metals and impurities.
Properties
Zinc is a bluish-white, lustrous metal with a low melting point and boiling point. It has a density of 7.14 g/cm3 and is relatively soft, making it easy to shape and work with.
Zinc is a reactive metal that easily combines with other elements and forms a wide variety of chemical compounds. It is moderately soluble in acids and alkalis, but reacts with non-metals such as oxygen and chlorine.
Zinc has excellent corrosion resistance due to the formation of a protective oxide layer on its surface. This layer prevents further oxidation and protects the metal from rust and other forms of corrosion. Zinc is often used as a coating for steel and other metals to improve their corrosion resistance.
Zinc is a good conductor of electricity and is often used as an electrode in batteries and other electrical applications.
Zinc is an essential element for human health and is required for many enzymatic processes in the body. It is also used in the production of vitamins and other nutritional supplements. However, excessive exposure to zinc can be toxic and can cause a range of health problems.
Applications
Galvanization
Zinc is commonly used to coat steel and iron to protect against corrosion. This process is known as galvanization and is used in a variety of applications, including construction, automotive, and electrical industries.
Alloys
Zinc is often used as an alloying element in the production of brass, which is a mixture of copper and zinc. Brass is used in a wide range of applications, including musical instruments, plumbing fixtures, and decorative items.
Batteries
Zinc is used in the production of batteries, particularly in zinc-carbon and alkaline batteries. These batteries are used in a variety of devices, including remote controls, flashlights, and toys.
Chemicals
Zinc is used in the production of a variety of chemicals, including zinc oxide, which is used in the production of rubber, ceramics, and glass. It is also used in the production of zinc stearate, which is used as a lubricant in the plastics and rubber industries.
Health and medicine
Zinc is an essential mineral that plays a key role in many bodily functions, including immune system function and wound healing. It is used in a variety of health and medicine applications, including dietary supplements, sunscreen, and antifungal creams.
Agriculture
Zinc is an important nutrient for plant growth and is used as a fertilizer in agricultural applications. It is also used in animal feed as a supplement to promote growth and improve health.
Interesting facts
The use of zinc can be traced back to ancient times, where it was used in a variety of applications such as creating brass, jewelry, and healing wounds. The first evidence of zinc smelting dates back to 500 BC in India.
Zinc is an essential mineral that is required for numerous biological functions in the human body, including immune system function, wound healing, and DNA synthesis. Zinc deficiency can lead to a variety of health problems, including impaired growth and development, weakened immune function, and delayed wound healing.
Zinc is highly resistant to corrosion, making it a popular choice for coating metals to protect them from rust and corrosion. This process is called galvanization, where zinc is electroplated onto steel to create galvanized steel.
Zinc is a popular choice for batteries due to its low cost and high energy density. Zinc-carbon batteries are commonly used in consumer electronics such as remote controls, while zinc-air batteries are used in hearing aids and other medical devices.
Zinc is highly recyclable, with up to 90% of zinc-containing products being recycled at the end of their lifecycle. Recycling zinc helps to conserve natural resources and reduce the environmental impact of zinc production.
Related
More elements
External links
- https://www.rsc.org/periodic-table/element/30/zinc
- https://en.wikipedia.org/wiki/Zinc
- https://www.britannica.com/science/zinc
- https://pubchem.ncbi.nlm.nih.gov/element/Zinc
- https://education.jlab.org/itselemental/ele030.html
- https://www.ducksters.com/science/chemistry/zinc.php
- https://www.chemicool.com/elements/zinc.html
- https://chemistrytalk.org/zinc-element/
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
