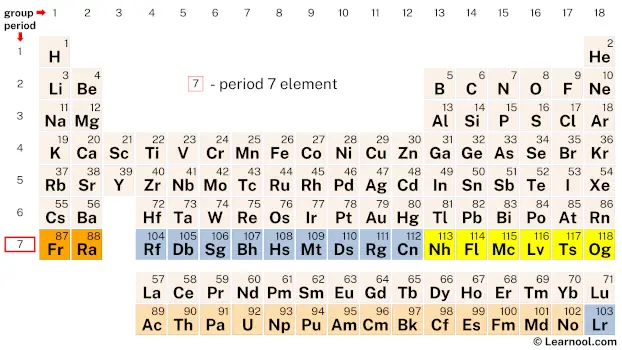
Period 7 elements refer to the chemical elements in the seventh row or period of the periodic table, ranging from atomic number 87 (francium) to atomic number 118 (oganesson). This period contains a total of 32 elements that are classified into four main categories: alkali metals, alkaline earth metals, actinides, and transition metals. In addition, elements with atomic numbers from 109 to 118 are synthetic and have unknown chemical properties, as they have been created artificially in laboratories.
Period 7 elements have unique physical and chemical properties due to their large atomic size and high electron density. The elements in this period have diverse uses in various fields, such as industrial applications, medical research, and nuclear power. Some of the elements found in period 7 include bohrium and seaborgium, which are highly unstable and difficult to study due to their short half-lives and reactivity. However, advancements in technology and experimental techniques have led to an increased understanding of the properties and behaviors of these elements. Further research on period 7 elements can lead to new discoveries and advancements in science and technology.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| 7 | – period 7 element |
Period 7 is the seventh row of elements on the periodic table and includes 32 elements, ranging from the highly reactive alkali metal Francium to the synthetic element Oganesson.
| Francium (Fr) | |
| Radium (Ra) | |
| Actinium (Ac) | |
| Thorium (Th) | |
| Protactinium (Pa) | |
| Uranium (U) | |
| Neptunium (Np) | |
| Plutonium (Pu) | |
| Americium (Am) | |
| Curium (Cm) | |
| Berkelium (Bk) | |
| Californium (Cf) | |
| Einsteinium (Es) | |
| Fermium (Fm) | |
| Mendelevium (Md) | |
| Nobelium (No) | |
| Lawrencium (Lr) | |
| Rutherfordium (Rf) | |
| Dubnium (Db) | |
| Seaborgium (Sg) | |
| Bohrium (Bh) | |
| Hassium (Hs) | |
| Meitnerium (Mt) | |
| Darmstadtium (Ds) | |
| Roentgenium (Rg) | |
| Copernicium (Cn) | |
| Nihonium (Nh) | |
| Flerovium (Fl) | |
| Moscovium (Mc) | |
| Livermorium (Lv) | |
| Tennessine (Ts) | |
| Oganesson (Og) | |
Element information
s-block
Francium

Francium is a highly reactive metal that belongs to the alkali metal group in the periodic table. It is a soft, silvery-white metal that is highly radioactive and has a short half-life. Francium is the second rarest naturally occurring element in the Earth’s crust, and its properties are poorly understood due to its scarcity and high radioactivity. It is a highly reactive element that can react with water and other halogens, and its compounds have very few practical applications.
Radium

Radium is a highly radioactive element that belongs to the alkaline earth metal group in the periodic table. It is a silvery-white metal that emits alpha, beta, and gamma rays and its longest lived isotope has a half-life of around 1600 years. Radium was discovered in 1898 and was once used extensively in luminous paints and medical treatments due to its radioactive properties. However, due to the health risks associated with its radioactivity, its use has been largely discontinued. Radium has no known biological function, and its compounds have very few practical applications.
f-block
Actinium

Actinium is a silvery-white, radioactive metal that belongs to the group of actinides. It was discovered in 1899 by French chemist André-Louis Debierne, who isolated it from pitchblende residue. Actinium is a soft metal that rapidly tarnishes in air, and it is highly radioactive. It has no stable isotopes and only exists in trace amounts in the earth’s crust. Due to its rarity and high radioactivity, actinium has limited commercial uses, but it has potential applications in medicine and nuclear technology.
Thorium
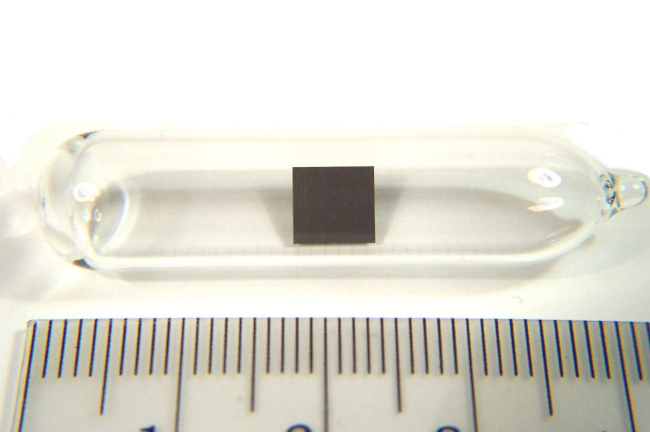
Thorium is a radioactive element that belongs to the group of actinides. It was discovered in 1828 by Swedish chemist Jöns Jacob Berzelius and is named after Thor, the Norse god of thunder. Thorium is a silvery-white metal that is malleable and ductile. It is the most stable of all the actinides and has several isotopes, one of which is widely used as a fuel in nuclear reactors. Thorium is also used in the production of high-quality lenses, as a component of gas mantles for lighting, and in various industrial applications. It has potential uses in nuclear technology as an alternative to uranium, as it is more abundant and generates less nuclear waste.
Protactinium
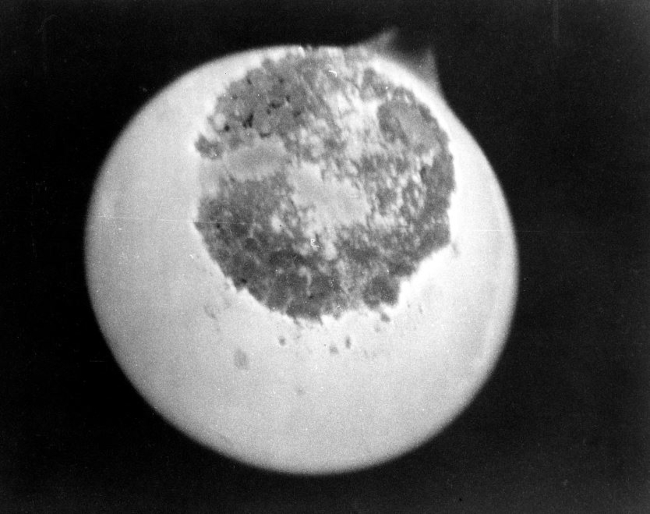
Protactinium is a radioactive and highly toxic element. It is a rare and silvery-gray metal that belongs to the actinide series and is found in trace amounts in uranium ores. Protactinium has a half-life of around 32,000 years and undergoes alpha decay to form actinium-227. Due to its scarcity and high radioactivity, protactinium has few practical uses, and is primarily used in basic scientific research to investigate its properties and behavior.
Uranium

Uranium is a silvery-white, weakly radioactive metal that belongs to the actinide series of elements. It is the heaviest naturally occurring element on Earth, and it is commonly known for its fissile isotope uranium-235, which is primarily used in nuclear power plants and weapons. Uranium also has some industrial uses, such as in the manufacture of alloys and as a coloring agent in ceramics. However, due to its radioactivity, it is highly regulated and requires special handling and storage.
Neptunium

Neptunium is a radioactive, silvery metal element belonging to the actinide series. It was discovered in 1940 by Edwin M. McMillan and Philip H. Abelson at the University of California, Berkeley. Neptunium is primarily produced as a byproduct of nuclear reactors and nuclear weapons testing. It has several isotopes, with neptunium-237 being the most stable one with a half-life of 2.14 million years. Neptunium is highly radioactive and toxic, and exposure to it can be harmful to humans.
Plutonium
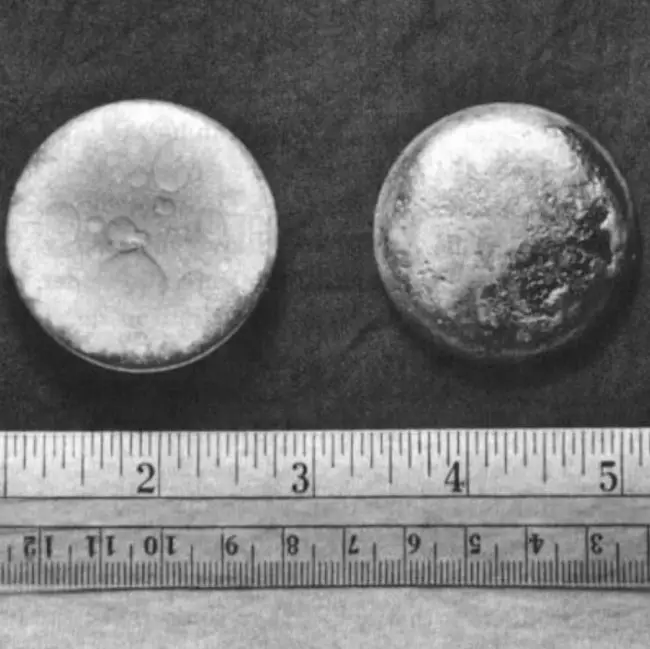
Plutonium is a highly radioactive, silvery metal element that belongs to the actinide series. It was first synthesized by Glenn T. Seaborg, Edwin M. McMillan, Joseph W. Kennedy, and Arthur C. Wahl at the University of California, Berkeley in 1940. Plutonium is primarily produced as a byproduct of nuclear reactors and nuclear weapons testing. It has several isotopes, with plutonium-239 being the most commonly used one for nuclear weapons and energy production. Plutonium is highly radioactive and toxic, and exposure to it can be harmful to humans. Due to its radioactive nature and potential use in nuclear weapons, strict regulations are in place for handling, transportation, and disposal of plutonium.
Americium

Americium is a radioactive metal that is commonly used as a source of ionizing radiation in industry and medicine. It was first synthesized by Glenn T. Seaborg and his colleagues in 1944 as part of the Manhattan Project. Americium is a silvery-white metal that slowly tarnishes in air, and it has relatively high melting and boiling points. It is highly radioactive and emits alpha particles, beta particles, and gamma rays, which can be harmful to human health if not handled properly. Despite its potential dangers, americium has various applications, including in smoke detectors, medical equipment, and nuclear weapons.
Curium

Curium is a radioactive, silvery-white metal that was first synthesized by Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944. It is named after Marie and Pierre Curie, the pioneers of radioactivity. Curium has few practical applications, but it is used in scientific research to study the properties of heavy elements and nuclear reactions. Its isotopes have half-lives ranging from several hours to millions of years, depending on the specific isotope. The longest-lived isotope, curium-247, has a half-life of over 15 million years. Curium isotopes can be used as sources of alpha particles in radioisotope thermoelectric generators (RTGs) that are used to convert the heat generated by the decay of radioactive isotopes into electricity.
Berkelium
Berkelium is a synthetic radioactive element. It is a member of the actinide series and belongs to the group of transuranium elements. It was first synthesized in 1949 by a team of scientists led by Glenn T. Seaborg at the University of California, Berkeley. Berkelium is highly radioactive and has no known biological role. Its longest-lived isotope has a half-life of over 1,300 years. Berkelium is primarily used for research purposes and in the production of other synthetic elements.
Californium
Californium is a synthetic radioactive element, with the atomic number 98. It is a member of the actinide series and belongs to the group of transuranium elements. It was first synthesized in 1950 by a team of scientists Stanley Gerald Thompson, Kenneth Street Jr., Albert Ghiorso, and Glenn T. Seaborg at the University of California, Berkeley. Californium is highly radioactive and has no known biological role. Its longest-lived isotope has a half-life of 898 years. Californium is primarily used for research purposes, such as in nuclear reactors and as a neutron source.
Einsteinium
Einsteinium is a member of the actinide series and belongs to the group of transuranium elements. It was first synthesized in 1952 by a team of scientists led by Albert Ghiorso at the University of California, Berkeley. Einsteinium is highly radioactive and has no known biological role. Its most stable isotope has a half-life of 471 days. Einsteinium is primarily used for research purposes, such as in the production of other synthetic elements and as a neutron source.
Fermium
Fermium is a radioactive transuranic element belonging to the actinide series. It was named after Enrico Fermi, an Italian physicist, and was first synthesized in 1952. Fermium does not occur naturally and is produced by bombarding lighter elements with neutrons. It has no known biological role and is highly toxic. Due to its short half-life, fermium has few practical uses, and its main application is in scientific research.
Mendelevium
Mendelevium is a synthetic element that was first synthesized in 1955 by a team of scientists at the Lawrence Berkeley National Laboratory. It is named after the Russian chemist Dmitri Mendeleev, who is known for his work on the periodic table of elements. Mendelevium is a highly radioactive metal that is produced by bombarding a target material with alpha particles. Its most stable isotope has a half-life of only 51 days. Due to its short half-life and high radioactivity, mendelevium has no practical applications and is mainly used for basic scientific research, particularly in the fields of nuclear physics and chemistry.
Nobelium
Nobelium is a synthetic element and a member of the actinide series. It was named after Alfred Nobel, the inventor of dynamite. Nobelium does not occur naturally and is produced by bombarding curium with carbon or nitrogen ions. It is highly radioactive and has no known biological role. Nobelium’s properties are not well studied, and its only practical use is in scientific research.
d-block
Lawrencium
Lawrencium is a synthetic element. It is a member of the actinide series and is named after Ernest O. Lawrence, the inventor of the cyclotron particle accelerator. Lawrencium is a radioactive metal and is produced by bombarding lighter elements with heavy ions. Its longest-lived isotope, lawrencium-266, has a half-life of just 11 hours. Because of its high radioactivity and short half-life, there are no known practical applications of lawrencium.
Rutherfordium
Rutherfordium is a synthetic element with the symbol Rf. It is a member of the transactinide series and is named after the physicist Ernest Rutherford. Rutherfordium is a radioactive metal and is produced by bombarding lighter elements with heavy ions. Its most stable isotope, rutherfordium-267, has a half-life of less than an hour. Because of its high radioactivity and short half-life, there are no known practical applications of rutherfordium.
Dubnium
Dubnium is a synthetic element with the atomic number 105. It was first synthesized in 1970 by Soviet scientists at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia. Dubnium is highly radioactive, and all of its isotopes are unstable and decay rapidly, with the longest-lived isotope having a half-life of just 16 hours. Dubnium’s properties are not well known, and its only practical use is in scientific research, particularly in the study of nuclear reactions and the properties of heavy elements.
Seaborgium
Seaborgium, with the symbol Sg, is a synthetic element that was first synthesized in 1974. It belongs to the transactinide series of elements and is located in group 6 of the periodic table. Seaborgium is highly radioactive and has a very short half-life; this makes it difficult to study its properties. However, it is believed to have similar chemical properties to tungsten and molybdenum due to its electronic configuration.
Bohrium
Bohrium, also known as element 107, was first synthesized in 1981. It is a synthetic element with the symbol Bh and belongs to the transactinide series of elements. Bohrium is highly radioactive and has a very short half-life, which makes it difficult to study its properties. Due to its instability, its chemical properties are not well known, but it is believed to be a transition metal.
Hassium
Hassium, with the symbol Hs, is a synthetic element that was first synthesized in 1984. It is a superheavy element located in group 8 of the periodic table. Hassium is highly radioactive and also has a very short half-life, which makes it difficult to study its properties, along with seaborgium and bohrium. Its chemical properties are not well known, but it is believed to have similar properties to osmium and ruthenium due to its electronic configuration.
Meitnerium
Meitnerium is a synthetic element with the atomic number 109, which means that it is a member of the period 7 elements. It was named after the Austrian physicist Lise Meitner. Meitnerium is a highly radioactive element, and very little is known about its properties due to its short half-life. It is believed to be a solid metal at room temperature and have similar properties to those of iridium.
Darmstadtium
Darmstadtium is a synthetic element with the atomic number 110. It is a member of the period 7 elements and was named after the city of Darmstadt in Germany, where it was first synthesized. Darmstadtium is a highly radioactive element and is believed to be a solid metal at room temperature. Because of its short half-life, very little is known about its chemical and physical properties.
Roentgenium
Roentgenium is a synthetic element with the atomic number 111, and it is a member of the period 7 elements. It was named after Wilhelm Conrad Röntgen, a German physicist who discovered X-rays. Roentgenium is a highly radioactive element, and very little is known about its properties due to its short half-life. It is believed to be a solid metal at room temperature and have similar properties to those of copper.
Copernicium
Copernicium is an artificially produced element and one of the heaviest elements known. It was first synthesized in 1996 by a German research team, and its atomic number is 112. Due to its extremely short half-life, very little is known about the chemical properties of this element. However, it is believed to be a highly reactive metal that may share similar properties with zinc, cadmium, and mercury.
p-block
Nihonium
Nihonium is a synthetic element with an atomic number of 113. It was first synthesized in 2004 by a Japanese research team. Due to its short half-life, little is known about the chemical and physical properties of this element. However, it is believed to be a highly reactive metal, similar to other elements in its group, such as boron, aluminum, and gallium.
Flerovium
Flerovium is a synthetic element that was first synthesized in 1999 by a team of Russian and American scientists. Due to its short half-life, very little is known about the chemical properties of this element. However, it is believed to be a highly reactive metal that may share similar properties with lead and tin. Its name honors the Russian physicist Georgy Flyorov.
Moscovium
Moscovium is a highly radioactive element that belongs to the category of superheavy elements. Moscovium was first synthesized in 2003 by a joint research team from the Joint Institute for Nuclear Research in Russia and Lawrence Livermore National Laboratory in California. It was named after the Moscow Oblast, the region where the Joint Institute for Nuclear Research is located. Moscovium has no known biological role and has only been produced in very small quantities for scientific research purposes.
Livermorium
Livermorium has the atomic number 116 and the symbol Lv. It is another highly radioactive element that belongs to the category of superheavy elements. Livermorium was first synthesized in 2000 by a joint research team from the Joint Institute for Nuclear Research in Russia and Lawrence Livermore National Laboratory in California. It was named after the Lawrence Livermore National Laboratory, the institution where it was discovered. Livermorium has no known biological role and has only been produced in very small quantities for scientific research purposes.
Tennessine
Tennessine is a synthetic element with the symbol Ts. It is classified as an unknown chemical property and is part of the halogen group on the periodic table. The element was first synthesized in 2010 by a joint team of Russian and American scientists. Due to its short half-life, the element has no practical applications and is primarily studied for scientific purposes.
Oganesson
Oganesson is a highly unstable and synthetic element, which was first synthesized in 2002. It is the heaviest element currently known to science, with an atomic number of 118. Due to its high atomic number, oganesson is expected to be a member of the noble gas family, although its chemical properties are still unknown. Because of its extreme instability, oganesson has a very short half-life, meaning that it rapidly decays into other elements through a process called alpha decay. Oganesson is an interesting area of study for scientists, as they continue to learn more about this elusive and rare element.
Occurrence and distribution
Many of the period 7 elements are very rare and can only be found in trace amounts in the Earth’s crust. Some, like francium, are so unstable that they cannot be isolated and studied directly. Others, like uranium, are more abundant and have important practical applications in fields like nuclear energy. These elements are also found in the stars and in interstellar gas clouds, where they are formed through nuclear fusion and supernova explosions.
Properties
Physical properties
Period 7 elements have several physical properties that distinguish them from other elements. They are all metals. However, the elements from atomic number 109 to 118 are unknown chemical properties and are all synthetic. These elements generally follow the trend of decreasing atomic size and increasing non-metallic character from left to right across the period. They have high melting and boiling points due to the strong metallic bonding between atoms, and they tend to be dense, with a high specific gravity. Some of the elements in this period, like francium and seaborgium, are radioactive, while others, such as bohrium and hassium, have not been studied enough to determine their melting and boiling points.
Chemical properties
Period 7 is made up of actinides and superheavy elements. Due to their large size and high atomic number, the period 7 elements exhibit a wide range of chemical properties. They tend to have a variety of oxidation states, and many of them are radioactive. Some notable period 7 elements include uranium, plutonium, and americium, which are commonly used in nuclear power plants and weapons.
Electronic configuration
The electron configuration of period 7 elements is characterized by the filling of the 7s, 6d, and 5f orbitals, which can hold up to two, ten, and fourteen electrons, respectively. However, not all period 7 elements have their 5f orbitals fully occupied due to the effect of relativistic contraction, which causes a decrease in the size of the orbitals and makes them more tightly bound to the nucleus. For instance, the 5f orbitals of actinides such as curium and berkelium are only partially filled, while those of the later actinides become progressively more contracted and filled until reaching lawrencium, which has a fully filled 5f orbital.
Related
More topics
- Period 1 element
- Period 2 element
- Period 3 element
- Period 4 element
- Period 5 element
- Period 6 element
- Period 7 element
External links
- Period 7 element – Wikipedia
- Period 7 element Facts for Kids – Kids encyclopedia facts
- Period 7 Elements: The Actinides – Chemistry LibreTexts
- Period 7 element – Elements Wiki | Fandom
- Periodic Table’s 7th Period is Finally Complete, IUPAC-IUPAP Officials Say – Sci.News
- Period 7 element – wikidoc
- About: Period 7 element – DBpedia
- Period 7 Element – Academic Accelerator
- What are the f block elements in period 7 known as? – Socratic
- 7th Period Of The Periodic Table Complete – Hackaday
- How come there are only 26 elements in period 7? – Quora
- Period 7 – Science Learning Hub
- what is different about most of the elements of the 7th period? – Brainly
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
