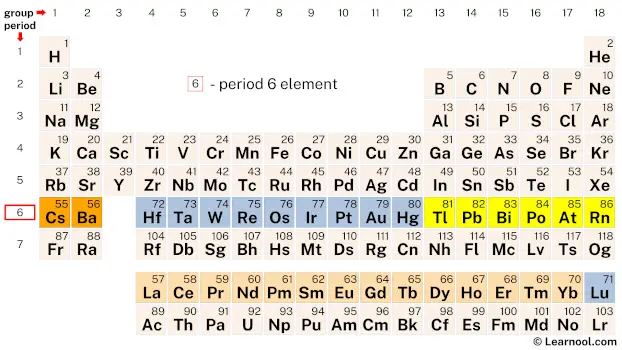
Period 6 elements refer to the chemical elements found in the sixth row or period of the periodic table, starting from the element cesium (Cs) and ending with the element radon (Rn). This group of elements exhibits a wide range of chemical and physical properties, which makes them important in various industrial, medical, and technological applications.
Period 6 elements also exhibit interesting magnetic and optical properties. For example, elements such as gadolinium and europium are known for their strong magnetic moments, which make them useful in the production of magnets and MRI contrast agents. Other elements, such as tungsten and osmium, have unique physical properties that make them useful in various industrial applications. For instance, tungsten has the highest melting point of all metals and is used in the production of filaments for incandescent light bulbs and X-ray tubes. Osmium, on the other hand, is the densest naturally occurring element and is used in the production of electrical contacts, fountain pen nibs, and phonograph needles. The study and exploration of these properties in period 6 elements hold great potential for the development of new technologies and materials in various fields.
Period 6 elements include a diverse group of metals, nonmetals, and metalloids, with a wide range of oxidation states and electron configurations. Many of these elements are essential components of life, such as sulfur, which is a crucial element in amino acids, and iodine, which is important in the production of thyroid hormones. Their unique properties make period 6 elements a fascinating area of research and development in the field of chemistry.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| 6 | – period 6 element |
Period 6 of the periodic table consists of 32 elements, including the commonly known elements such as gold and platinum, as well as the less familiar elements such as lutetium, thulium, and erbium.
| Caesium (Cs) | |
| Barium (Ba) | |
| Lanthanum (La) | |
| Cerium (Ce) | |
| Praseodymium (Pr) | |
| Neodymium (Nd) | |
| Promethium (Pm) | |
| Samarium (Sm) | |
| Europium (Eu) | |
| Gadolinium (Gd) | |
| Terbium (Tb) | |
| Dysprosium (Dy) | |
| Holmium (Ho) | |
| Erbium (Er) | |
| Thulium (Tm) | |
| Ytterbium (Yb) | |
| Lutetium (Lu) | |
| Hafnium (Hf) | |
| Tantalum (Ta) | |
| Tungsten (W) | |
| Rhenium (Re) | |
| Osmium (Os) | |
| Iridium (Ir) | |
| Platinum (Pt) | |
| Gold (Au) | |
| Mercury (Hg) | |
| Thallium (Tl) | |
| Lead (Pb) | |
| Bismuth (Bi) | |
| Polonium (Po) | |
| Astatine (At) | |
| Radon (Rn) | |
Element information
s-block
Cesium

Cesium is a highly reactive, silvery-gold alkali metal that is the first member in period 6 of the periodic table. It has a very low melting point and is the softest metal. Cesium is highly reactive and can spontaneously ignite in air, which is why it is usually stored in an inert gas. It is used in atomic clocks, as well as in the oil drilling industry.
Barium

Barium is a soft, silvery-white alkaline earth metal that is the second element in period 6 of the periodic table. Barium has a low melting point and is highly reactive, similar to other elements in its group. It is used in the production of numerous products, including fireworks, glass, and rubber. Barium sulfate is used as a contrast agent in medical X-rays due to its ability to absorb X-rays. It also has industrial applications in the production of lubricants and paints. However, barium and its compounds are toxic and should be handled with care.
f-block
Lanthanum

Lanthanum is a silvery-white, ductile, and malleable metal that belongs to the group of rare-earth elements. It has the atomic number 57 and is the first element in the lanthanide series. Lanthanum has a relatively low melting point and boiling point compared to most metals. It is a good electrical conductor and is used in various electronic devices, including camera lenses, X-ray screens, and ignition elements for lighters. Lanthanum compounds are also used in catalysts for petroleum refining, glass manufacturing, and as an alloying agent in some steels.
Cerium

Cerium is a soft, silvery-white metal that belongs to the lanthanide series. It has the atomic number 58 and is the second element in the series. Cerium has a low melting point and is highly reactive, especially when exposed to air. It has a variety of applications, including in catalytic converters for automobiles, glass polishing, and in alloys for making lighter flints. Cerium compounds are also used in the production of self-cleaning ovens and phosphors for fluorescent lamps.
Praseodymium

Praseodymium is a rare-earth metal that belongs to the lanthanide series of elements. It is soft, silvery-white, and highly reactive, quickly tarnishes in air. It has a hexagonal close-packed crystal structure and is paramagnetic at room temperature. Praseodymium is used in alloys with magnesium to create high-strength metals for aircraft engines and in high-power magnets for use in motors, headphones, and other electronic devices.
Neodymium
Neodymium is another rare-earth metal and a member of the lanthanide series of elements. It is a hard, silvery metal that tarnishes slowly in air. It has a cubic crystal structure and is paramagnetic at room temperature. Neodymium is used in the manufacturing of powerful magnets, such as those found in computer hard drives, loudspeakers, and motors. It is also used in the production of glass, as a component of didymium glass, which is used to create certain types of lenses, such as those found in welding goggles.
Promethium
Promethium is a rare-earth metal that is highly radioactive and has no stable isotopes. It is a soft silvery-white metal that tarnishes rapidly in air and is easily oxidized. Along with niobium, promethium is one of only two elements in the periodic table that are named after a mythological character. Promethium is named after the Greek Titan Prometheus, while niobium is named after Niobe, the daughter of Tantalus in Greek mythology. Its compounds have various applications, including as a phosphorescent material and in nuclear batteries.
Samarium

Samarium is another rare-earth metal that is silvery-white in color and has a rhombohedral crystal structure. It is a relatively stable element and has five stable isotopes. Samarium is used in the manufacture of permanent magnets, as well as in nuclear reactors to control the rate of neutron reactions. Its compounds also have various industrial applications, including in catalysts, electronic ceramics, and optical materials.
Europium

Europium is a rare-earth metal that has a silvery-white appearance and is soft and ductile. It is primarily used in the manufacture of red and blue phosphors for color TV tubes and computer monitors. It is also used as a dopant in some types of glass to create a yellow or red coloring. Europium has a high melting point and is chemically reactive; it readily oxidizes in air. It is also a good conductor of electricity.
Gadolinium

Gadolinium is a rare-earth metal that has a silvery-white appearance and is relatively soft and ductile. It has unusual magnetic properties, making it useful in various magnetic resonance imaging (MRI) applications. It is also used as a neutron absorber in nuclear power plants and as a contrast agent in MRI scans. Gadolinium has a high melting point and is chemically reactive; it readily reacts with water to form hydrogen gas. It is also a good conductor of electricity.
Terbium

Terbium is a soft, silvery-white, rare-earth element that belongs to the lanthanide series. It is named after the village of Ytterby in Sweden, where it was first discovered. Terbium is used in the production of electronic devices, such as solid-state devices, and in the production of green phosphors used in fluorescent lamps and TV tubes. It is also used as a dopant in fiber optic amplifiers and as a contrast agent in medical imaging.
Dysprosium

Dysprosium is a rare-earth metal that belongs to the lanthanide series. It is silvery-white in color and has a metallic luster. Dysprosium is used in the production of permanent magnets, which are used in electric motors, generators, and other applications. It is also used in nuclear reactors as a neutron absorber, and in lighting applications as a component of metal-halide lamps. Dysprosium oxide is used in the production of ceramic capacitors for use in electronic devices.
Holmium

Holmium is a rare-earth metal and is one of the least abundant of the lanthanides. It has a silver-gray appearance and is relatively soft and malleable. Holmium has the highest magnetic moment of any naturally occurring element and is used in a variety of magnetic applications. It also has a relatively high specific heat, making it useful in alloys for high-temperature applications. Holmium oxide is used as a yellow and red coloring agent for glass and ceramics.
Erbium

Erbium is a rare-earth metal that has a bright, silvery appearance. It is relatively soft and ductile and has a high melting point. Erbium is used as a dopant in fiber optic amplifiers and as a coloring agent in glass and ceramics. It also has a number of medical applications, such as in the treatment of certain types of cancer. Erbium’s electron configuration makes it unique in that it has an anomalously high melting point for a lanthanide element.
Thulium

Thulium is a rare, silvery-white metal belonging to the lanthanide series of elements. It has a high melting point and is soft and malleable, which makes it easy to shape. Thulium has a few applications in industry, such as being used as a radiation source for portable X-ray machines and as a dopant in fiber optic amplifiers.
Ytterbium

Ytterbium is a soft, silvery metal belonging to the lanthanide series of elements. It has a relatively low melting point and is both ductile and malleable. Ytterbium is commonly used in atomic clocks due to its exceptional accuracy, and also has applications in nuclear medicine and as a radiation source in portable X-ray machines. In addition, ytterbium is used as a dopant in fiber optic amplifiers and as a catalyst in organic chemical reactions.
d-block
Lutetium

Lutetium is a silvery-white metal and is the last element in the lanthanide series. It has a relatively high melting point and is a rare-earth element that is difficult to extract and purify. Lutetium is often used as a catalyst in various chemical reactions and is also used in nuclear medicine for imaging and therapy. It has only one stable isotope, and its radioactive isotopes have relatively long half-lives, which makes it useful for certain medical and industrial applications. Lutetium compounds are also used in the production of superconductors and as a dopant in optical materials.
Hafnium

Hafnium is a lustrous, silvery-gray metal that belongs to the transition metals group. It is found in minerals such as zircon and is used in various applications due to its high melting point, corrosion resistance, and ability to absorb neutrons. Hafnium is widely used in the nuclear industry, particularly in the control rods of nuclear reactors, as it has a high capacity for absorbing neutrons. It is also used in gas turbines, aerospace applications, and electrical components due to its good thermal and electrical conductivity.
Tantalum

Tantalum is a silvery-gray, dense metal with a melting point of 3017 ℃, which makes it one of the most refractory metals. It has excellent corrosion resistance, high strength, and good ductility. Tantalum is widely used in the electronics industry due to its ability to form stable oxide layers and its resistance to corrosion by acids. It is also used in alloys for aircraft parts, missile components, and surgical implants.
Tungsten

Tungsten, also known as wolfram, is a dense, steel-gray metal with the highest melting point of any element, at 3422 ℃. It is extremely hard, brittle, and has high tensile strength. Tungsten has the lowest coefficient of thermal expansion of any pure metal and has excellent thermal and electrical conductivity. It is commonly used in light bulb filaments, X-ray tubes, and other high-temperature applications. Tungsten is also used in alloys for drill bits, armor-piercing projectiles, and aerospace components.
Rhenium

Rhenium is a dense, silvery-white metal that is extremely resistant to heat and wear. It has the third highest melting point of all the elements and is one of the densest transition metals. It is widely used as an alloying agent in high-temperature alloys for aerospace applications and in electrical contacts.
Osmium

Osmium is a hard, brittle, bluish-white metal with a very high melting point. It is the densest of all naturally occurring elements and is one of the least abundant elements in the Earth’s crust. Osmium is used in alloys with other metals to make fountain pen tips, electrical contacts, and instrument pivots, and in the production of filaments for light bulbs and electrical equipment.
Iridium

Iridium is a dense, hard, silvery-white metal with a very high melting point. It is the most corrosion-resistant metal known and is used in a variety of applications, including as a hardening agent for platinum alloys, in spark plugs, and in crucibles for growing large, high-purity single crystals. Iridium is also used in the aerospace industry due to its high strength and resistance to heat and wear.
Platinum

Platinum is a dense, silvery-white metal with a high melting point and excellent resistance to corrosion. It is a highly valued precious metal and is used in jewelry, electrical contacts, laboratory equipment, and catalytic converters for vehicles. Platinum is also used in a variety of industrial applications, including in the production of fertilizers, plastics, and explosives. Due to its high conductivity and resistance to corrosion, platinum is also used as an electrode material in fuel cells.
Gold

Gold is a chemical element that is soft, dense, and shiny with a bright yellow color. It is one of the least reactive chemical elements, making it an excellent conductor of electricity and resistant to corrosion. Gold is a precious metal that has been used for various purposes throughout history, including currency, jewelry, and decoration.
Mercury

Mercury is a heavy, silvery metal that is liquid at room temperature. It possesses a unique property: the ability to form alloys with most other metals, due to which it has a wide range of applications, such as in thermometers and barometers. However, mercury is also highly toxic and poses a significant risk to human health and the environment if not handled properly. Its use has been significantly reduced in recent years due to health and safety concerns.
p-block
Thallium

Thallium is a soft, malleable, and ductile metal with a silvery-white appearance that tarnishes to a bluish-gray color upon exposure to air. It has a variety of isotopes, some of which are radioactive and have been used as a neutron source in nuclear reactors. Thallium is a toxic element that can cause serious health effects, particularly to the nervous system, and its use and disposal are regulated in many countries. Thallium is mainly used in electronic components, infrared optical equipment, and in some medical applications.
Lead

Lead is a heavy, soft, bluish-gray metal that is highly toxic. It is used in a variety of applications, including batteries, ammunition, and radiation shielding. Lead has been widely used in pipes, plumbing fixtures, and paint, but its use in these applications has been significantly reduced due to its health hazards. Lead poisoning can cause a range of health effects, including developmental delays, cognitive impairment, and behavioral problems.
Bismuth

Bismuth is a heavy, dense, silvery-white metal that is relatively stable in air and water. It has a low thermal conductivity and a high electrical resistance, making it useful in some applications such as thermo-electric cooling devices. Bismuth is also commonly used in alloys with other metals, such as lead and tin, to improve their mechanical properties.
Polonium

Polonium is a rare and highly radioactive element that is extremely toxic. It is primarily produced through the decay of uranium and thorium, and is not found in nature in significant quantities. Polonium has a silvery-gray appearance and is one of the few elements that readily emits alpha particles, which are highly ionizing radiation. Due to its high toxicity and radioactivity, polonium has few practical applications and is mainly used for research purposes.
Astatine
Astatine is a rare and highly radioactive element. It is a member of the halogen family, and its properties are similar to those of iodine. It has no stable isotopes, and its most stable isotope 210At has a half-life of just 8.1 hours. Astatine is typically produced by bombarding bismuth with alpha particles. It is a very rare element, and only a few milligrams are produced each year.
Radon
Radon is a radioactive gas that is formed by the decay of uranium and thorium in rocks and soil. It is a member of the noble gas family and is the heaviest known gas. Radon is colorless, odorless, and tasteless, which makes its detection difficult without special equipment. It is highly radioactive and can cause lung cancer if inhaled in high concentrations. Radon is used in some medical treatments, such as radiation therapy for cancer, and is also used to calibrate radiation detectors.
Properties
Electronic configuration
Period 6 elements have their valence electrons in the 6s and 4f orbitals, which contributes to their unique chemical properties. The partially filled 4f orbitals of the lanthanides are responsible for their unique chemical properties, while the partially filled 5d orbitals of the transition metals in period 6 result in their reactivity. In general, the period 6 elements tend to form compounds with a +2 oxidation state due to the stability of the 6s orbital, although other oxidation states are also possible.
Atomic and ionic radii
The atomic and ionic radii of period 6 elements decrease as you move across the period from left to right. This trend can be attributed to the increasing positive charge of the nucleus, which attracts the electrons closer to the center of the atom and reduces the size of the atom. As a result, the atomic and ionic radii of these elements decrease across the period. The smaller atomic and ionic radii of these elements lead to higher ionization energies and electronegativities, which makes them less reactive and more likely to form stable compounds.
Melting and boiling point
The melting and boiling points of period 6 elements are generally high due to strong metallic bonding. This is particularly true for the transition metals, such as hafnium, tantalum, tungsten, and rhenium, which have high melting and boiling points due to strong metallic bonding resulting from the delocalization of electrons in their d orbitals. However, radon, which is a noble gas in this period, has low melting and boiling points due to its weak van der Waals forces between atoms.
Electronegativity
The electronegativity of period 6 elements tends to increase as you move across the period from left to right. This trend is due to the increasing nuclear charge, which attracts electrons more strongly and makes it more difficult to remove them. As a result, the period 6 elements tend to be more electronegative and more likely to attract electrons in chemical reactions.
Related
More topics
- Period 1 element
- Period 2 element
- Period 3 element
- Period 4 element
- Period 5 element
- Period 6 element
- Period 7 element
External links
- Period 6 element – Wikipedia
- Period 6 Elements: The Lanthanides – Chemistry LibreTexts
- About: Period 6 element – DBpedia
- Period 6 Element – Academic Accelerator
- Period 6 element – wikidoc
- Period 6 element – Elements Wiki | Fandom
- Periodic Table: period 6 elements Flashcards – Quizlet
- The symbol and group number of the Period 6 transition element whose atoms have the fewest protons are? – Homework.Study.com
- What are the f-block elements in period 6 known as? – Socratic
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
