
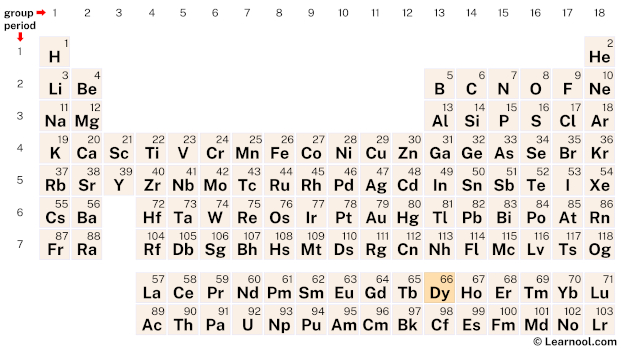
Dysprosium (Dy) is a chemical element of the periodic table, located in the period 6, and has the atomic number 66. It is the tenth element in the lanthanide series. It is a soft, lustrous, silvery-white metal, whose name comes from the Greek word “dysprositos”, which means hard to get. It is never found freely in nature and is counted as one of the rare earth elements.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – f block |
Dysprosium (Dy) is located on the periodic table in the lanthanide series, which is a group of elements located at the bottom of the table. Specifically, in period 6, between terbium (Tb) and holmium (Ho).
Element information
 |
|
 |
|
| Origin of name | Greek word “dysprositos” (which means hard to get) |
| Symbol | Dy |
| Atomic number (Z) | 66 |
| Atomic mass | 162.5 u |
| Block | f-block |
| Period | 6 |
| Classification | Lanthanide |
| Atomic radius | 178 pm |
| Covalent radius | 192±7 pm |
| Melting point | 1407 ℃, 2565 ℉, 1680 K |
| Boiling point | 2562 ℃, 4653 ℉, 2840 K |
| Electron configuration | [Xe] 4f10 6s2 |
| Learn how to write: Dysprosium electron configuration | |
| Electrons per shell | 2, 8, 18, 28, 8, 2 |
| Learn how to draw: Dysprosium Bohr model | |
| Crystal structure | Hexagonal close-packed (hcp) |
| Phase at r.t | Solid |
| Density near r.t | 8.540 g/cm3 |
| Main isotopes | Dysprosium-156, Dysprosium-158, Dysprosium-160, Dysprosium-161, Dysprosium-162, Dysprosium-163, Dysprosium-164 |
| Natural occurrence | Primordial |
| Oxidation state | +3 |
| Electronegativity (Pauling scale) | 1.22 |
| Protons Neutrons Electrons |
66 99 66 |
| CAS number | 7429-91-6 |
| Discovered by | Lecoq de Boisbaudran in 1886 |
History

Dysprosium is a rare earth element that was first discovered in 1886 by French chemist Paul Émile Lecoq de Boisbaudran. He was working with the mineral gadolinite, which had been previously discovered in a mine in Ytterby, Sweden. Dysprosium was isolated as an impurity in yttrium oxide (Y2O3), which had been extracted from gadolinite. The name “dysprosium” comes from the Greek word “dysprositos,” meaning “hard to get at,” which reflects the difficulty of separating dysprosium from other rare earth elements.
Dysprosium’s properties were not fully characterized until the early 20th century. In 1906, Carl Auer von Welsbach separated dysprosium from other rare earth elements and discovered that it had strong emission lines in the yellow region of the spectrum. This made it useful for color television tubes and other applications that required a bright yellow color.
In the mid-20th century, dysprosium became important for its magnetic properties. It has one of the highest magnetic moments of any element and is used in the production of powerful permanent magnets, especially in applications such as wind turbines and electric vehicles. Today, China is the world’s largest producer of dysprosium, followed by the United States and Australia.
Occurrence and production
Dysprosium is found in various minerals including xenotime, fergusonite, gadolinite, euxenite, and blomstrandine. The element is typically extracted from monazite sand, a mineral that contains varying amounts of thorium and other rare earth elements. The largest deposits of monazite sands are found in Brazil, India, China, and Malaysia.
The production of dysprosium is a complex process that involves a series of chemical and metallurgical steps. The first step in the production process involves the extraction of rare earth elements from monazite sand using various methods such as solvent extraction, ion exchange, and precipitation. Once the rare earth elements are extracted, they are further separated using techniques such as solvent extraction and ion exchange. Dysprosium is then separated from the other rare earth elements using ion exchange or solvent extraction methods. The purified dysprosium is then converted into various forms such as oxides, metals, and alloys, which can be used in various industrial applications.
The production of dysprosium is typically carried out in countries that have significant reserves of rare earth minerals, such as China, which is the world’s largest producer of rare earth elements. Other major producers of dysprosium include Australia, the United States, and Russia. The production of dysprosium has become increasingly important in recent years due to its use in various high-tech applications, including electric vehicles, wind turbines, and other renewable energy technologies.
Properties
Dysprosium is a rare earth element that belongs to the lanthanide series of the periodic table.
Dysprosium is a soft, silvery-white metal that tarnishes quickly when exposed to air.
It has a relatively high melting point of 1407 ℃ and a boiling point of 2562 ℃.
Dysprosium is paramagnetic, which means that it is weakly attracted to magnetic fields.
It is also highly reactive with water and acids, and can ignite spontaneously in air.
Dysprosium has seven stable isotopes with atomic masses ranging from 156 to 164. The most stable of which is dysprosium-154 with a half-life of approximately 3 million years.
Applications
Dysprosium is a key component in the production of high-performance magnets used in wind turbines, hybrid vehicles, and other industrial applications.
Dysprosium is used in the production of specialized lighting, such as high-intensity discharge lamps, which are commonly used in streetlights and stadium lighting.
Dysprosium is used in the control rods of nuclear reactors to regulate the rate of fission reactions.
Dysprosium is used in the production of magneto-optical disks, which are used for data storage in some computer systems.
Dysprosium is used in magnetic resonance imaging (MRI) as a contrast agent to enhance the visibility of certain tissues and organs.
Dysprosium is used in glass polishing compounds, which are used to remove scratches and blemishes from glass surfaces.
Interesting facts
Dysprosium has one of the highest magnetic moments of any element, making it useful for producing strong permanent magnets.
The element was named after the Greek word “dysprositos,” which means “hard to get at,” as it was initially difficult to extract in pure form.
Dysprosium is a relatively abundant rare earth element, with an average abundance in the Earth’s crust of 5.2 parts per million.
Dysprosium is used in nuclear reactors to absorb neutrons and regulate nuclear reactions.
Dysprosium oxide has a unique pink color, which is used in the production of ceramic glazes.
Dysprosium has the highest thermal neutron absorption cross-section of any element, making it useful in nuclear reactors and for detecting thermal neutrons.
Dysprosium has been used in lighting applications, such as compact fluorescent bulbs, due to its ability to emit a bright white light when excited by an electric current.
Dysprosium isotopes have been used in medical research to study bone growth and metabolism.
Related
More elements
External links
- https://www.rsc.org/periodic-table/element/66/dysprosium
- https://en.wikipedia.org/wiki/Dysprosium
- https://www.britannica.com/science/dysprosium
- https://www.chemicool.com/elements/dysprosium.html
- https://pubchem.ncbi.nlm.nih.gov/element/Dysprosium
- https://www.livescience.com/38292-dysprosium.html
- https://study.com/academy/lesson/what-is-dysprosium-used-for-common-industrial-uses.html
- https://www.thoughtco.com/dysprosium-facts-element-66-or-dy-4125571
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
