A metal is a material that is characterized by its luster, malleability, ductility, and conductivity. These properties are the result of the metallic bond, which is the electrostatic attraction between positively charged metal ions and a sea of delocalized electrons. Metals are widely used in construction, manufacturing, and other applications due to their strength, durability, and versatility. They are also used in numerous everyday items, such as jewelry, coins, and electrical wires.
The term metal is derived from the Greek word “metallon,” which means “mine” or “quarry.” The use of metals can be traced back to the earliest known civilizations, with the discovery of copper dating back to around 9000 BC. Over time, humans learned to extract and use other metals, such as gold, silver, and iron, and these metals became increasingly important for economic and technological development.
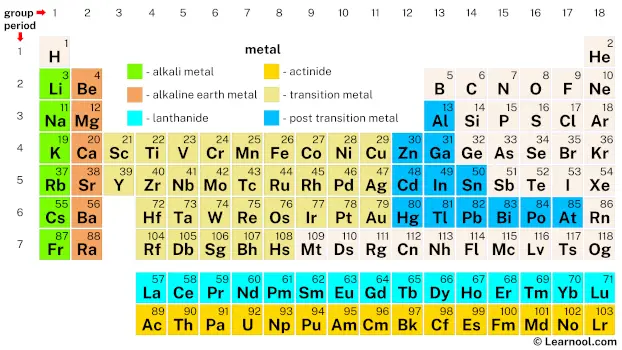
In the modern era, metals have been organized into the periodic table based on their atomic structure and properties. The periodic table is a tabular arrangement of the chemical elements, arranged in order of increasing atomic number. Metals make up the majority of the periodic table, with 85 out of the 118 known elements being classified as metals. The elements are organized into groups and periods, with metals occupying the majority of the left side and center of the table.
The metals in the periodic table are divided into two categories: alkali metals, which are highly reactive and have one valence electron, and alkaline earth metals, which are less reactive and have two valence electrons. The transition metals, located in the center of the table, have multiple valence electrons and exhibit a wide range of properties. Other categories of metals include the post-transition metals, lanthanides, and actinides.
The study of metals is an important field of science and engineering, with applications in materials science, metallurgy, and other related disciplines. As our understanding of metals and their properties continues to evolve, so too does our ability to harness their unique characteristics for new and innovative applications in a wide range of fields.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – alkali metal | – actinide | ||
| – alkaline earth metal | – transition metal | ||
| – lanthanide | – post-transition metal | ||
Metals in the periodic table include alkali metals, alkaline earth metals, lanthanides, actinides, transition metals, and post-transition metals. Out of the 118 known elements in the periodic table, 85 are classified as metals.
History

Metals have been used for thousands of years, with copper being one of the earliest metals to be discovered and used by humans. The use of metals evolved over time, with bronze (an alloy of copper and tin) being developed in the Bronze Age and iron being introduced in the Iron Age. These metals were used for weapons, tools, and decorative objects, and their widespread use contributed to the development of ancient civilizations.
The use of metals led to the development of metalworking techniques, including forging, casting, and soldering. These techniques were used by ancient civilizations such as the Egyptians, Greeks, and Romans, who created intricate metal objects for both practical and decorative purposes. Metalworking also played a significant role in religious and cultural practices, with metal objects being used in religious ceremonies and as symbols of power and authority.
The discovery and extraction of new metals continued throughout history, with the discovery of gold and silver in the Americas during the 16th century leading to a major economic boom. Advances in metallurgy during the Industrial Revolution led to the development of new techniques for extracting and refining metals, which made it possible to produce large quantities of metals for use in machinery, transportation, and other industries.
The Industrial Revolution brought about significant changes in the production and use of metals, with new manufacturing processes and machinery leading to increased production and efficiency. Metallurgy became a distinct field of study during this time, with scientists and engineers working to develop new alloys and improve manufacturing processes. The study of metals is fundamental in both science and engineering because of their diverse applications across various industries and technologies.
Categories
Metals can be classified into different categories based on their properties and placement in the periodic table. The majority of elements in the periodic table are metals, with 85 out of the 118 known elements being classified as metals. The placement of metals in the periodic table helps to determine their properties and behavior. The periodic table organizes elements into different categories, including alkali metals, alkaline earth metals, lanthanides, actinides, transition metals, and post-transition metals. Each category has distinct properties and characteristics that help to determine their behavior.
The alkali metals are a group of highly reactive metals located in group 1 of the periodic table. They are soft, silvery-white metals that readily react with water and other elements, such as oxygen and halogens. Alkali metals have only one valence electron, making them highly reactive and more likely to lose an electron to form positive ions.
Alkaline earth metals are located in group 2 of the periodic table and are less reactive than alkali metals. They have two valence electrons and are harder and denser than alkali metals. These metals are also used in various applications, such as construction and the production of batteries.
The lanthanides and actinides are two series of elements located at the bottom of the periodic table. The lanthanides are also known as rare-earth elements and are used in various applications such as electronics, magnets, and lighting. The actinides, on the other hand, are mostly synthetic and have nuclear applications, such as nuclear reactors and weapons.
The transition metals are a group of elements located in the center of the periodic table, with multiple valence electrons and exhibiting a wide range of properties. These metals are typically strong, dense, and have high melting and boiling points. They are also good conductors of heat and electricity and are widely used in various industries, such as manufacturing, construction, and electronics.
The post-transition metals are elements located to the right of the transition metals in the periodic table, with less metallic character and exhibiting more nonmetallic behavior. These metals have lower melting and boiling points compared to transition metals, and they are typically softer and less dense. Some examples of post-transition metals include aluminum, tin, and lead.
Classification
In addition to the categories of metals based on their placement in the periodic table, metals can also be classified into various groups based on their properties and usage. Some common classifications include ferrous metals, non-ferrous metals, precious metals, heavy metals, light metals, rare-earth metals, and superalloys.
Ferrous metals
Ferrous metals are metals that contain iron as the primary constituent. They are known for their strength and durability, making them popular in construction and manufacturing industries. Examples of ferrous metals include iron, steel, and cast iron. Iron is the most abundant and widely used metal in the world, and is used to produce steel, which is an alloy of iron and carbon. Cast iron, on the other hand, is an alloy of iron, carbon, and silicon, and is used for making pipes, cookware, and engine blocks.
Non-ferrous metals
Non-ferrous metals are metals that do not contain iron as the primary constituent. They are often lighter and more malleable than ferrous metals, and have a higher resistance to corrosion. Examples of non-ferrous metals include copper, aluminum, zinc, nickel, lead, and tin. Copper is used for electrical wiring and plumbing, while aluminum is used for making aircraft frames and soda cans. Zinc is often used as a coating for iron and steel to protect against corrosion, while nickel is used for making coins and batteries.
Precious metals

Precious metals are rare and valuable metals that are often used for jewelry and currency. They are known for their luster and resistance to corrosion. Examples of precious metals include gold, silver, and platinum. Gold is a popular investment and is used for making jewelry, coins, and bullion. Silver is also used for making jewelry and coins, as well as in photography and electronics. Platinum is a dense and durable metal that is used for making jewelry and catalytic converters.
Heavy metals
Heavy metals are metals that have a high density and atomic weight. They are often toxic and can accumulate in the environment and in living organisms. Examples of heavy metals include mercury, lead, and cadmium. Mercury is a liquid metal that is used in thermometers and fluorescent light bulbs, but is also a neurotoxin. Lead is used in batteries, pipes, and ammunition, but can cause lead poisoning. Cadmium is used in batteries and coatings, but can cause kidney damage and cancer.
Light metals
Light metals are metals that have a low density and are typically lightweight. They are used in a variety of applications where weight is a crucial factor. The most common light metals are aluminum, magnesium, and titanium. These metals have excellent strength-to-weight ratios and are highly resistant to corrosion.
Aluminum is the most abundant metal on earth and is widely used in various industries, such as construction, transportation, and packaging. It is lightweight, strong, and highly resistant to corrosion, making it an ideal material for making aircraft and other lightweight structures.
Magnesium is the lightest metal and is also highly resistant to corrosion. It is used in the aerospace industry, as well as in the manufacture of electronics and other consumer products.
Titanium is known for its strength and corrosion resistance, as well as its lightweight properties. It is commonly used in the aerospace industry, as well as in the manufacture of medical implants and other high-performance applications.
Rare-earth metals
Rare-earth metals are a group of 17 chemical elements that are vital to many modern technologies, including electronics, computer systems, and green energy technologies. They are typically found in low concentrations in the earth’s crust and are difficult to extract and refine.
The most common rare-earth metals include scandium, yttrium, and lanthanum. These metals have unique properties that make them valuable in a variety of applications. Scandium is used in the production of high-intensity lights and aerospace alloys, while yttrium is used in LED lighting, lasers, and superconductors. Lanthanum finds use in catalysts, camera lenses, and steel production.
Superalloys
Superalloys are a group of metals that have excellent strength and high-temperature resistance. They are used in a variety of high-performance applications, such as in jet engines, gas turbines, and nuclear reactors.
The most common super alloys include Inconel, Hastelloy, and Waspaloy. These alloys are typically made up of a combination of nickel, cobalt, iron, and other metals. They are highly resistant to corrosion and can withstand extreme temperatures and pressures.
Properties
Luster and conductivity
Metals are characterized by their luster, which is their ability to reflect light. This property is due to the free electrons in the metal atoms, which are able to absorb and re-emit light. Metals also have high electrical conductivity, which means they can easily conduct electricity. This property is due to the presence of free electrons in the metal, which can move freely through the metal lattice and carry electrical charge.
Malleability and ductility
Metals are also known for their ability to be hammered into thin sheets (malleability) and drawn into thin wires (ductility). These properties are due to the metallic bond, which allows metal atoms to move and slide past each other without breaking the bond. This allows metals to be shaped into a wide variety of forms, making them useful in many different applications.
Strength and durability
Metals are generally strong and durable materials, which makes them useful for construction, manufacturing, and other applications where toughness and resilience are important. This property is due to the strong metallic bond between the atoms, which allows the metal to resist deformation and fracture.
Corrosion and oxidation resistance
Many metals are susceptible to corrosion and oxidation, which can weaken the material and make it less useful. However, some metals are more resistant to these processes than others, and may be used in applications where exposure to the elements is common. For example, stainless steel is a corrosion-resistant alloy that is commonly used in kitchen appliances and medical devices.
Magnetic and electrical properties
Some metals are magnetic, meaning they can be attracted to a magnet. This property is due to the arrangement of electrons in the metal atoms, which can create a magnetic field. Metals also have unique electrical properties, which may be useful in certain applications. For example, some metals have a high resistance to electrical current, which makes them useful as electrical wiring materials.
Applications
Construction

Metals such as steel and aluminum are commonly used in construction for their strength, durability, and resistance to corrosion. They are used in a variety of structures, including buildings, bridges, and infrastructure.
Transportation
Metals play a crucial role in the transportation industry, where their strength, low weight, and resistance to corrosion are highly valued. Cars, trucks, trains, and airplanes all rely on metals for their frames, engines, and other components.
Electronics
Metals like copper, silver, and gold are excellent conductors of electricity, making them essential components in electrical wiring, circuitry, and electronic devices. They are also used in batteries, solar cells, and other renewable energy technologies.
Manufacturing
Metals are used in manufacturing a wide range of products, from machinery and tools to household appliances and consumer electronics. Their strength, durability, and machinability make them ideal for use in high-stress applications.
Jewelry and art
Precious metals like gold, silver, and platinum are highly valued for their beauty and rarity, making them popular materials for jewelry and decorative art. Other metals like copper and bronze are also used in sculpture and other forms of art.
Medicine
Metals play an important role in medicine, where they are used in everything from surgical instruments and implants to X-ray and MRI machines. They are also used in the production of pharmaceuticals and other medical products.
Agriculture
Metals like iron and copper are used in agriculture for a variety of purposes, including tools, machinery, and fertilizers. They are also used in the production of food packaging and storage containers.
Related
More topics
- Block (periodic table)
- Group (periodic table)
- Period (periodic table)
- Metal
- Alkali metal
- Alkaline earth metal
- Lanthanide
- Actinide
- Transition metal
- Post-transition metal
- Metalloid
- Nonmetal
- Reactive nonmetal
- Noble gas
External links
- Metal Definition & Meaning – Merriam-Webster
- Metal – Wikipedia
- Metal | Definition, Characteristics, Types, & Facts – Britannica
- METAL | definition in the Cambridge English Dictionary – Cambridge Dictionary
- What is a Metal ? – University of Washington
- Metal Definition & Meaning – Dictionary.com
- Explainer: What is a metal? – Science News Explores
- Metals, Metalloids, and Nonmetals – Angelo State University
- METAL definition in American English – Collins Dictionary
- What elements are metals? – Socratic
- Periodic Table Metals and Non-Metals – ChemTalk
- Periodic Table Metals | Definition, Reactivity & Examples – Study.com
- List of Metals – Science Notes and Projects
- The Periodic Table: Metals, Nonmetals, and Metalloids – Dummies
- 4.2: Metals, Metalloids and Nonmetals – Chemistry LibreTexts
- Metals and non-metals in the periodic table – The Royal Society
- Elements & Periodic Table: Metals – Chem4Kids
- Basic Types of Metals on the Periodic Table – YourDictionary
- Metals, Nonmetals, and Metalloids of the Periodic Table – ThoughtCo
- Metals and Non Metals of the Periodic Table – Breaking Atom
- Metals and the Periodic Table – Springer
- What is the total number of metals in the periodic table? – Quora
- What are metals and non-metals on the periodic table? – BBC
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
