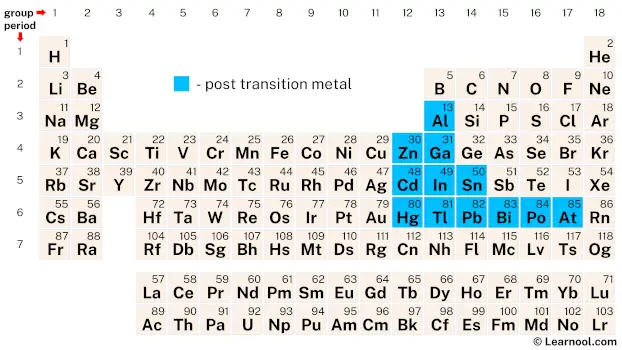
Post-transition metals, also known as poor metals, are a group of chemical elements located in the periodic table between the transition metals and the metalloids. They are called poor metals because they have some of the characteristics of both metals and nonmetals, and often exhibit properties that are intermediate between the two.
The post-transition metals, which include elements such as aluminum, zinc, gallium, cadmium, indium, tin, mercury, thallium, lead, bismuth, and polonium, are typically found in groups 12 to 17 of the periodic table. It’s worth noting, however, that the classification of astatine, another element in this group, is not always straightforward. While it’s generally considered a post-transition metal, some authors may classify it as a nonmetal or a metalloid.
These elements are referred to as “post-transition” because they follow the transition metals in the periodic table, and because their chemistry is distinct from that of the transition metals. The post-transition metals are generally softer, have lower melting and boiling points, and are less dense than the transition metals. They are also less reactive and have a tendency to form covalent bonds rather than ionic bonds.
The post-transition metals have a wide range of uses in industry and technology. For example, aluminum is used extensively in construction and transportation, while zinc is used to galvanize steel to protect it from corrosion. Thallium and mercury are used in the production of electronic devices and chemicals, while indium is used in the production of touchscreens and solar panels. Cadmium is used in rechargeable batteries, and lead is used in ammunition and radiation shielding. Polonium has niche uses in the nuclear industry due to its radioactive properties.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – post-transition metal |
The post-transition metals in the periodic table include 12 chemical elements from group 12 to group 17: aluminum, zinc, gallium, cadmium, indium, tin, mercury, thallium, lead, bismuth, polonium, and astatine.
History

The post-transition metals were discovered and named in the late 18th and early 19th centuries. Aluminum, for example, was first isolated by Danish chemist Hans Christian Ørsted in 1825, while zinc was first identified as an element by Andreas Marggraf in 1746. The discovery of gallium is attributed to French chemist Paul-Émile Lecoq de Boisbaudran, who isolated the metal in 1875. Cadmium was discovered in 1817 by German chemist Friedrich Stromeyer, while indium was first identified spectroscopically by Ferdinand Reich and Hieronymous Theodor Richter in 1863.
Tin has a long history of use by humans, dating back to the Bronze Age. Mercury was also known to the ancients, and was used by the Greeks and Romans in various applications. Thallium was discovered spectroscopically by Sir William Crookes in 1861. Lead has been known since ancient times, and was used by the Romans in plumbing and other applications. Bismuth was first recognized as a distinct element by French chemist Claude François Geoffroy in 1753. Polonium was discovered in 1898 by Marie and Pierre Curie, while astatine was first synthesized in 1940 by Dale R. Corson, Kenneth Ross MacKenzie, and Emilio G. Segrè.
Applications
Aluminum
Used in the construction industry for making frames, siding, roofs, and gutters.
Used in the transportation industry for making car parts, airplanes, and boats.
Used in the packaging industry for making cans, foils, and lids.
Zinc
Used in galvanizing steel to protect against corrosion.
Used in batteries, particularly for hearing aids and watches.
Used in the rubber industry as a vulcanizing agent.
Gallium
Used in electronic devices such as LEDs and solar cells.
Used in the production of semiconductors.
Used as a coating on mirrors and other reflective surfaces.
Cadmium
Used in batteries, particularly in nickel-cadmium batteries.
Used in pigments and coatings for plastics and metals.
Used in nuclear reactors as a neutron-absorbing material.
Indium
Used in electronic devices such as LCD screens, touch screens, and solar cells.
Used in semiconductors and transistors.
Used as a thin film coating for glass to improve conductivity.
Tin
Used in coating steel to prevent corrosion (tinplate).
Used in making alloys such as bronze and pewter.
Used in soldering and as a component in dental amalgam.
Mercury
Used in thermometers and barometers.
Used in dental fillings (amalgam).
Used in electrical switches and relays.
Thallium
Used in medical imaging for detecting heart disease.
Used in electronic devices such as photocells and infrared detectors.
Used in making lenses for special glasses and prisms.
Lead
Used in batteries, particularly in lead-acid batteries.
Used in the construction industry for roofing, plumbing, and paint.
Used in ammunition and radiation shielding.
Bismuth

Used in cosmetics, particularly in lipstick and eye shadow.
Used in pharmaceuticals for treating stomach ulcers.
Used in alloys such as pewter and low-melting-point solders.
Polonium
Used in nuclear reactors as a neutron source.
Used in some industrial processes to eliminate static electricity.
Used as a heat source in space equipment like radioisotope thermoelectric generators (RTGs).
Astatine
Limited practical applications due to its rarity and radioactivity.
Has been used in some medical applications, such as cancer treatment.
Has potential uses in nuclear medicine and scientific research.
Interesting facts
Post-transition metals generally have lower melting and boiling points than transition metals, making them easier to work with in certain applications, such as casting and soldering.
Many post-transition metals have been known since ancient times, except for gallium, cadmium, indium, thallium, polonium, and astatine, which were first discovered in the late 1800s.
Aluminum is the most abundant metal in the Earth’s crust and is used in various applications due to its strength, light weight, and resistance to corrosion.
Mercury is the only post-transition metal that is liquid at room temperature.
Thallium is a highly toxic metal that was used in rat poison but is now strictly regulated due to its harmful effects on human health.
Lead was once widely used in gasoline, paint, and plumbing but is now known to be toxic to humans and the environment.
Polonium, a radioactive metal that emits alpha particles, gained notoriety for its use in the poisoning of Russian dissident Alexander Litvinenko in 2006.
Astatine is the rarest naturally occurring element on Earth and has a half-life of only a few hours, making it difficult to study.
Related
More topics
- Block (periodic table)
- Group (periodic table)
- Period (periodic table)
- Metal
- Alkali metal
- Alkaline earth metal
- Lanthanide
- Actinide
- Transition metal
- Post-transition metal
- Metalloid
- Nonmetal
- Reactive nonmetal
- Noble gas
External links
- Post-Transition Metals – Diamond Light Source
- Post-transition metal – Wikipedia
- 8.6: Group 13 (and a note on the post-transition metals) – Chemistry LibreTexts
- Post-Transition Metals – Chemistry Learner
- Elements – Post-transition, Poor, Other Metals – Ducksters
- Q: What are the elements classified as post transition metals? – CK-12 Foundation
- Post-transition metal – Elements Wiki | Fandom
- Periodic Table of the Elements – Post-transition Metals – Cool Periodic Table
- Zinc, Cadmium, and Mercury: The Post-Transition Metals – Wondrium Daily
- The periodic table – transition metals (video) – Khan Academy
- Post-transition metal – Chem Europe
- Post-Transition Metal – an overview – ScienceDirect
- Characterizing the Elements – Los Alamos National Laboratory (.gov)
- Post Transition Metal – Academic Accelerator
- Transition Metals – Angelo State University
- Periodic Table: Post-Transition Metals – LibGuides
- Transition metal | Definition, Properties, Elements, & Facts – Britannica
- Post transition metals – Isaac’s science blog
- post-transition metal – Wiktionary
- Post-Transition Metals – Fisher Scientific
- Post-Transitional Metal: Definition – Breaking Atom
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
