
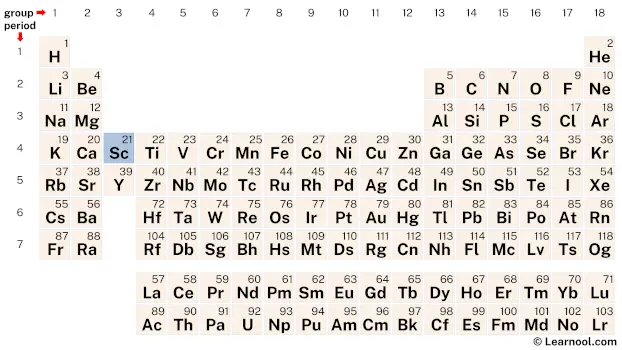
Scandium (Sc) is a chemical element of the periodic table, located in the group 3 and the period 4, and has the atomic number 21. It is a soft, silvery-white transition metal, whose name comes from the Latin word “Scandia”, which means Scandinavia. It is counted as one of the rare earth elements.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – d block |
Scandium is a d-block element, found in the third column and the fourth row of the periodic table. It has the atomic number 21 and is denoted by the symbol Sc.
Element information
 |
|
 |
|
| Origin of name | Latin word “Scandia” (which means Scandinavia) |
| Symbol | Sc |
| Atomic number (Z) | 21 |
| Atomic mass | 44.955912 u |
| Block | d-block |
| Group | 3 |
| Period | 4 |
| Classification | Transition metal |
| Atomic radius | 162 pm |
| Covalent radius | 170±7 pm |
| Van der Waals radius | 211 pm |
| Melting point | 1541 ℃, 2806 ℉, 1814 K |
| Boiling point | 2836 ℃, 5136 ℉, 3109 K |
| Electron configuration | [Ar] 3d1 4s2 |
| Electrons per shell | 2, 8, 9, 2 |
| Learn how to draw: Scandium Bohr model | |
| Crystal structure | Hexagonal close-packed (hcp) |
| Phase at r.t | Solid |
| Density near r.t | 2.985 g/cm3 |
| Main isotopes | Scandium-45 |
| Natural occurrence | Primordial |
| Oxidation state | +3 |
| Electronegativity (Pauling scale) | 1.36 |
| Protons Neutrons Electrons |
21 24 21 |
| Learn how to find: Scandium protons neutrons electrons | |
| CAS number | 7440-20-2 |
| Discovered by | Lars Fredrik Nilson in 1879 |
History

Scandium was discovered by Swedish chemist Lars Fredrik Nilson in 1879, while he was analyzing rare minerals from Scandinavia. Nilson suspected that he had discovered a new element, but he was unable to isolate it in pure form. In 1880, Nilson’s fellow countryman Per Teodor Cleve succeeded in isolating scandium from the minerals euxenite and gadolinite.
Scandium’s existence and properties had been predicted by Russian chemist Dmitri Mendeleev in his periodic table, which he had published in 1869. Mendeleev had left gaps in his table to accommodate undiscovered elements, and he had predicted the properties of these elements based on their position in the table. He named the missing element ekaboron, and predicted that it would have chemical properties similar to those of aluminum.
When scandium was discovered, it was found to have many of the properties that Mendeleev had predicted for ekaboron. The element was named after Scandinavia, the region of Europe where it was discovered.
Occurrence and production
Scandium is not found in nature as a free element, but it is widely distributed in minerals. Its abundance in the Earth’s crust is about 22-25 ppm (parts per million).
Scandium is mainly found in two types of ores: thortveitite and wolframite. It is also present in some other minerals, such as bazzite, euxenite, and gadolinite. However, the amounts of scandium in these minerals are typically very low.
Scandium is produced mainly as a byproduct during the processing of various ores, such as uranium, tungsten, and rare earth elements. The production of scandium is still relatively small due to its low abundance and the high costs associated with its extraction and processing.
There are several methods used for the production of scandium, including the extraction from ores, purification of scandium-containing solutions, and electrolytic refining of scandium metal. One of the most common methods is to extract scandium from the red mud generated during the processing of bauxite for the production of alumina. The red mud contains significant amounts of scandium and other rare earth elements, which can be extracted by various chemical methods.
Another source of scandium is from the spent catalysts used in the refining of crude oil. These catalysts contain a small amount of scandium and other transition metals, which can be recovered by pyrometallurgical and hydrometallurgical methods.
In recent years, several new methods for the production of scandium have been developed, including the use of ion exchange resins and solvent extraction techniques. These methods offer the possibility of producing scandium at a lower cost and in larger quantities, which could lead to an increase in the use of scandium in various applications.
Properties
Scandium is a silvery-white metal that is relatively soft and lightweight.
It has a melting point of 1541 ℃ and a boiling point of 2836 ℃.
Scandium is paramagnetic, meaning it is weakly attracted to a magnetic field.
It is a fairly reactive metal, but it resists corrosion due to a thin layer of oxide that forms on its surface when exposed to air.
Scandium has a relatively high melting point and is a good conductor of electricity and heat.
Scandium is often used as an alloying element with aluminum to improve the strength and durability of aluminum alloys.
Scandium oxide is a highly refractory material that is used in the production of high-intensity lamps and lasers.
Scandium has a very low abundance in the Earth’s crust, making it a relatively rare element.
Scandium has a variety of isotopes, with the most stable being 46Sc, which has a half-life of 83 days.
Applications
Aerospace industry
Scandium alloys are used in aircraft components, including landing gear, jet engine parts, and aerospace structural materials. The addition of scandium increases the strength and durability of aluminum alloys.
Solid oxide fuel cells
Scandium-doped zirconia is used as an electrolyte in solid oxide fuel cells. Scandium increases the ionic conductivity of the electrolyte and improves the fuel cell’s performance.
Sports equipment
Scandium-aluminum alloys are used in sports equipment such as baseball bats, lacrosse sticks, and bicycle frames. The lightweight and high-strength properties of scandium make it an ideal material for these applications.
Lighting
Scandium is used in the manufacture of high-intensity lamps such as metal halide lamps, which are used in stadium and street lighting. The addition of scandium to these lamps increases their energy efficiency, brightness, and color rendering capabilities.
Electronics
Scandium oxide is used as a substrate for high-temperature superconducting thin films in electronic devices such as microwave filters and magnetic sensors.
Medical applications
Scandium-46 is used in positron emission tomography (PET) imaging to diagnose and monitor various diseases, including cancer and heart disease.
Glass manufacturing
Scandium oxide is added to glass to improve its durability and resistance to high temperatures, making it ideal for use in high-performance windows and lenses.
Interesting facts
Scandium was the first element to be discovered by spectral analysis.
Scandium is a rare element, found in small quantities in over 800 minerals.
Scandium has been used to make high-intensity lamps for studios and movie sets.
Scandium is used in the aerospace industry for lightweight construction of airplanes and rockets.
Scandium alloyed with aluminum can improve the strength and durability of sports equipment such as bicycle frames and baseball bats.
Scandium has a unique ability to absorb hydrogen, which has led to research into its potential use for hydrogen storage in fuel cells.
Scandium is also being investigated for its potential medical applications, such as cancer treatment and bone regeneration.
Related
More elements
External links
- https://en.wikipedia.org/wiki/Scandium
- https://www.rsc.org/periodic-table/element/21/scandium
- https://www.britannica.com/science/scandium
- https://www.chemicool.com/elements/scandium.html
- https://pubchem.ncbi.nlm.nih.gov/element/Scandium
- https://www.livescience.com/29071-scandium.html
- https://www.ducksters.com/science/chemistry/scandium.php
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
