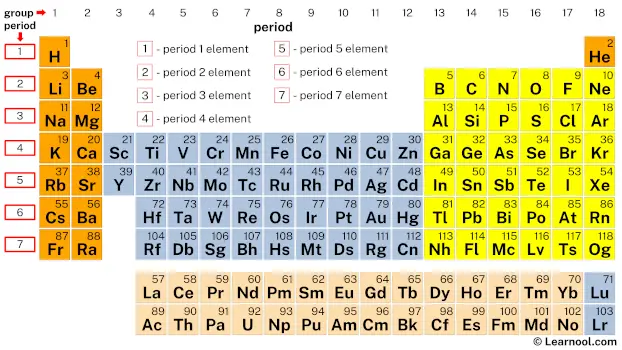
A period in the periodic table is a horizontal row of elements that share the same number of electron shells. In total, there are seven periods in the periodic table, each containing a different number of elements. The number of elements in a period corresponds to the number of electrons that can occupy the electron shells of atoms in that period.
The first period of the periodic table contains only two elements, hydrogen and helium. These two elements have only one electron shell and are therefore classified as belonging to the first period. The second period contains eight elements, from lithium to neon, which have two electron shells. Each subsequent period contains one additional electron shell, with the seventh period containing the elements with the largest electron shells known to date.
Elements within a period exhibit periodic trends in their properties, including decreasing atomic radius and increasing ionization energy and electronegativity from left to right. The elements within a period also exhibit similar chemical properties due to the number of valence electrons in their outermost electron shell.
The concept of periods in the periodic table was first proposed by Russian chemist Dmitri Mendeleev in the late 1800s. By arranging the elements in order of increasing atomic number and placing elements with similar properties in the same column, Mendeleev created a comprehensive system that allowed for the prediction of undiscovered elements and their properties. The periodic table has since become one of the most fundamental and useful tools in chemistry to provide insight into the behavior and properties of the elements.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| 1 | – period 1 element | 5 | – period 5 element |
| 2 | – period 2 element | 6 | – period 6 element |
| 3 | – period 3 element | 7 | – period 7 element |
| 4 | – period 4 element | ||
From top to bottom, the periodic table consists of 7 periods, with each period containing a different number of elements: period 1 has 2 elements, period 2 and period 3 have 8 elements each, period 4 and period 5 have 18 elements each, and period 6 and period 7 have 32 elements each, which makes a total of 118 known elements.
Period 1
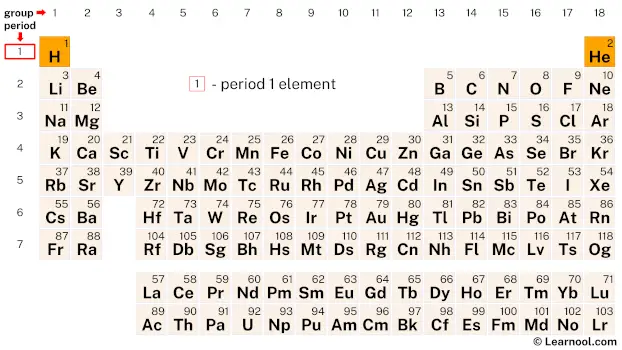
Period 1 is the first row of elements in the periodic table, consisting of only two elements, hydrogen (H) and helium (He).
Hydrogen
Hydrogen is the lightest and most abundant element in the universe, and also the most abundant element in the Earth’s crust. It is highly reactive and can form covalent, ionic, or metallic bonds with other elements. Hydrogen gas is used in various industrial processes, such as in the production of ammonia and methanol. It is also used as a fuel for rockets and in fuel cells for generating electricity.
Helium
Helium is an inert gas and is the second lightest element after hydrogen. It is the most stable of all the noble gases, and is the second most abundant element in the universe. Helium gas is used in various applications, such as in cooling systems for MRI machines and nuclear reactors, in airships and balloons, and as a shielding gas in welding processes.
Period 2
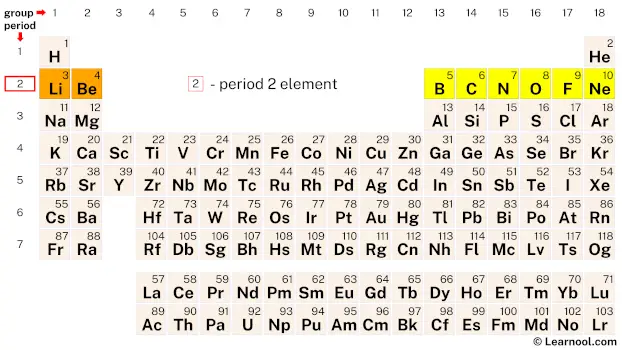
Period 2 of the periodic table contains eight elements, namely lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon.
Lithium
Lithium (Li) is a soft, silvery-white metal that belongs to the alkali metal group. It is highly reactive and has the lowest density of all metals. Lithium is commonly used in batteries, ceramics, and as a mood stabilizer in psychiatric medicine.
Beryllium
Beryllium (Be) is a hard, grayish metal that belongs to the alkaline earth metals. It has a high melting point and is used in nuclear reactors, aerospace applications, and X-ray equipment.
Boron
Boron (B) is a metalloid that has a high melting point and low density. It is used in a variety of applications, such as in ceramics, glass, and detergents.
Carbon
Carbon (C) is a nonmetallic element that is essential for life. It exists in several allotropes, including graphite and diamond. Carbon is used in a wide range of applications, such as in the production of steel and as a fuel source.
Nitrogen
Nitrogen (N) is a colorless, odorless gas that makes up about 78% of Earth’s atmosphere. It is an essential element for life and is used in the production of ammonia, which is used in fertilizers.
Oxygen
Oxygen (O) is a colorless, odorless gas that makes up about 21% of Earth’s atmosphere. It is essential for respiration and is used in a variety of applications, such as in steel production and medical oxygen tanks.
Fluorine
Fluorine (F) is a highly reactive, pale yellow gas that belongs to the halogen group. It is used in the production of uranium, as a refrigerant, and in the manufacture of high-performance plastics.
Neon
Neon (Ne) is a colorless, odorless gas that belongs to the noble gas group. It is used in neon lights, high-voltage indicators, and television tubes.
Period 3
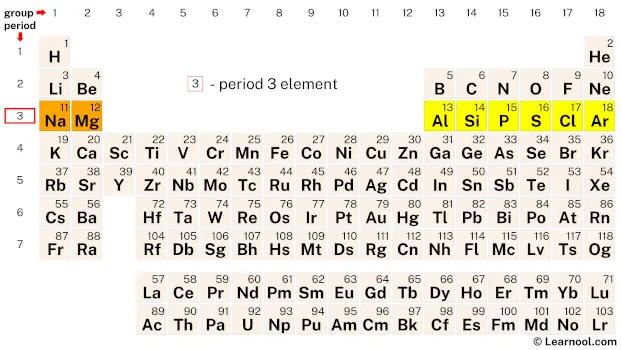
Period 3 refers to the third row of elements in the periodic table, consisting of sodium, magnesium, aluminum, silicon, phosphorus, sulfur, chlorine, and argon.
Sodium
Sodium (Na) is a soft, silvery-white, highly reactive metal. It is an essential element for animal life and is used in various applications, including the production of soap and as a coolant in nuclear reactors.
Magnesium
Magnesium (Mg) is a shiny gray metal that is widely used in alloys, medicine, and pyrotechnics. It is also an essential element for plants and animals, and is found in many foods such as nuts, seeds, and green leafy vegetables.
Aluminum
Aluminum (Al) is a lightweight, silvery-white metal that is widely used in various applications, including packaging, transportation, construction, and electrical systems. It is the most abundant metal in the Earth’s crust and is highly resistant to corrosion.
Silicon
Silicon (Si) is a metalloid that is widely used in the production of semiconductors, solar cells, and computer chips. It is the second most abundant element in the Earth’s crust and is also found in various minerals and rocks.
Phosphorus
Phosphorus (P) is a highly reactive, non-metallic element that is essential for life. It is found in DNA and is used in various applications, including fertilizers, detergents, and flame retardants.
Sulfur
Sulfur (S) is a non-metallic element that is found in various forms in the Earth’s crust. It is used in various applications, including the production of fertilizers, chemicals, and pharmaceuticals.
Chlorine
Chlorine (Cl) is a highly reactive, greenish-yellow gas that is used in various applications, including the production of bleach and disinfectants. It is also an essential element for life and is found in various compounds such as salt.
Argon
Argon (Ar) is an inert, colorless, odorless gas that is the third most abundant gas in the Earth’s atmosphere. It is used in various applications, including welding, lighting, and as a protective gas in various industrial processes.
Period 4
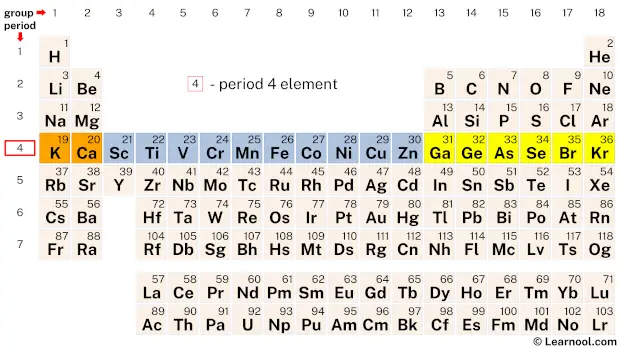
Period 4 of the periodic table includes eighteen elements, ranging from potassium (K) to krypton (Kr). These elements share some common characteristics due to their placement in the same period.
The first half of the period consists of elements that have a filling 3d subshell, such as scandium (Sc), titanium (Ti), and iron (Fe). These elements are known for their transition metal properties, such as high melting and boiling points, malleability, and good electrical conductivity. They are also commonly used in various industries, such as construction, aerospace, and electronics.
The second half of the period consists of elements that have a filling 4p subshell, such as germanium (Ge), selenium (Se), and bromine (Br). These elements exhibit more non-metallic properties, such as lower melting and boiling points, and increased electronegativity. They are commonly used in semiconductors, solar cells, and pharmaceuticals. The noble gas, krypton (Kr) is the last element in period 4 and is known for its inertness and use in lighting.
Period 5
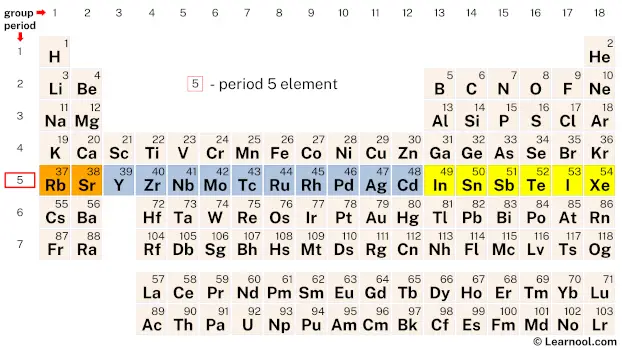
Period 5 of the periodic table contains 18 elements, beginning with the alkali metal rubidium (Rb) and ending with the noble gas xenon (Xe). Elements in period 5 generally have larger atomic radii and lower ionization energies compared to the elements in period 4, although their electron affinities are generally lower due to the trend of decreasing electron affinity down a group. This is due to the fact that the valence electrons in period 5 are in higher energy levels, farther away from the nucleus, and thus experience less attraction from the positively charged nucleus.
One notable characteristic of period 5 elements is their diverse range of chemical properties. For example, the transition metals in period 5, such as molybdenum (Mo) and technetium (Tc), have high melting and boiling points, and are often used as catalysts. On the other hand, iodine (I) is a highly reactive nonmetal belonging to the halogen group, while xenon (Xe) is an inert noble gas with very low reactivity.
Period 6
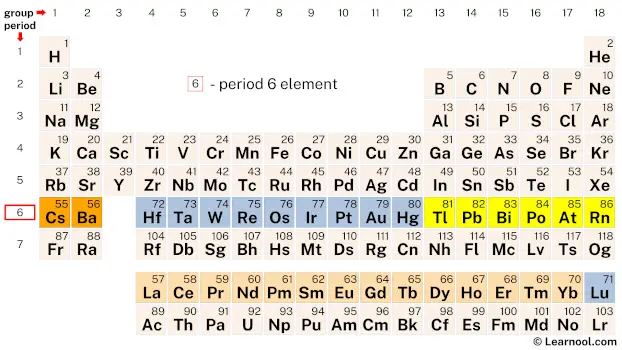
Period 6 of the periodic table contains 32 elements, starting with cesium (Cs) and ending with radon (Rn). This period includes some important elements such as barium (Ba), hafnium (Hf), tungsten (W), platinum (Pt), and gold (Au). These elements have important uses in industry, such as hafnium in nuclear reactors and tungsten in the manufacturing of high-temperature alloys. Others, such as gold and platinum, are highly valued for their beauty and rarity.
Period 7
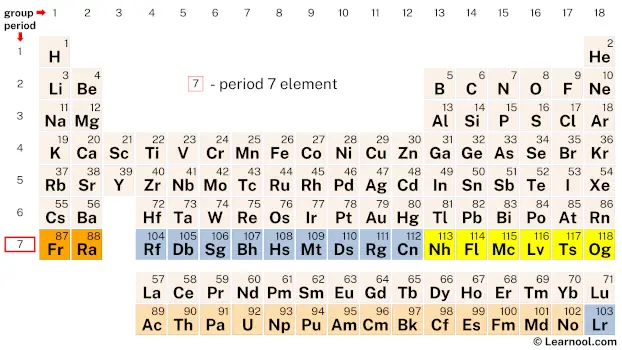
Period 7 of the periodic table contains 32 elements, starting with francium (Fr) and ending with oganesson (Og). The elements in this period have progressively increasing atomic numbers and are known for their unique chemical properties. Many of the elements in period 7 are synthetic and highly unstable, with short half-lives, making their study challenging. Elements such as uranium (U) and plutonium (Pu) are important in nuclear energy and have been the subject of extensive research. On the other hand, elements like francium (Fr) and oganesson (Og) are highly reactive and have limited practical applications.
Period 8
Period 8 elements are yet to be discovered, and therefore, their properties and characteristics are still unknown. The theoretical predictions suggest that the eighth period would consist of 18 elements, and their electronic configurations would be similar to the seventh period elements, with 5g orbitals filling up. Theoretical calculations also suggest that the elements of the eighth period would be extremely unstable and radioactive, and their lifetimes would be incredibly short.
The search for the elements of the eighth period is still ongoing, and scientists are actively working to synthesize these elements in laboratories using advanced technologies. However, due to the expected instability of these elements, it remains to be seen whether the predicted elements of the eighth period are physically possible. Until then, the properties and characteristics of the eighth period elements remain a mystery, and the possibility of the ninth period is still uncertain.
Related
More topics
- Block (periodic table)
- Group (periodic table)
- Period (periodic table)
- Metal
- Alkali metal
- Alkaline earth metal
- Lanthanide
- Actinide
- Transition metal
- Post-transition metal
- Metalloid
- Nonmetal
- Reactive nonmetal
- Noble gas
External links
- Period (periodic table) – Wikipedia
- 6.4: Modern Periodic Table- Periods and Groups – Chemistry LibreTexts
- Periodic table – Energy Kids – Energy Information Administration (.gov)
- The Structure and Meaning of the Periodic Table: Periods – Breaking Atom
- How to Read the Periodic Table | Groups & Periods – ChemTalk
- Definition of a Chemical Period – Chemistry Glossary – ThoughtCo
- Period | chemistry – Britannica
- Periodic Table Groups and Periods – Science Notes and Projects
- Elements & Periodic Table – Chem4Kids
- What are the periods and groups of the periodic table? How are they used? – Quora
- Reading the Periodic Table – California State University, Northridge
- Periodic Table Groups vs. Periods | Properties & History – Study.com
- What is a “period” of the periodic table? – Chemistry Stack Exchange
- Question Video: Determining Which Period of the Periodic Table an Element Is Found In – Nagwa Limited
- Periodic Table of Elements – Live Science
- Periods in the Periodic Table | Secondaire – Alloprof
- The Periodic Table: Families and Periods – Dummies
- How many periods are in the periodic table? – Socratic
- How to Read the Periodic Table — Overview & Components – Expii
- Periodic Table: Periods, Groups, and Families – Chemistry Learner
- Modern Periodic Table – CK-12
- Modern Periodic Table: Periods and Groups – Course Hero
- Period (periodic table) Facts for Kids – Kids encyclopedia facts
- How to Read the Periodic Table: 14 Steps (with Pictures) – wikiHow
- Periods in Periodic table Explained! (With 1-7 Period Names) – Periodic Table Guide
- Elements of periodic table are arranged into groups/periods – Mammoth Memory
- About: Period (periodic table) – DBpedia
- What do the periods in the periodic table tell us? – Brainly
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
