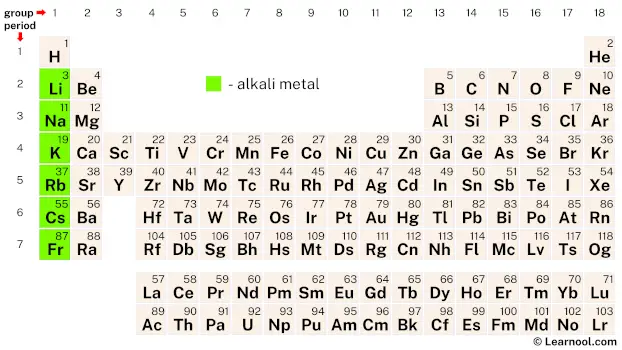
Alkali metals (also known as the lithium family) are a group of six elements on the periodic table that include lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). These elements are highly reactive and are located in the first column of the periodic table. They have one valence electron, which makes them highly reactive and easily lose their outermost electron to form positive ions. Due to their reactivity, they are never found as pure elements in nature but always exist as compounds.
The discovery of alkali metals dates back to the early 19th century when Humphry Davy, an English chemist, successfully isolated sodium by electrolysis in 1807. The same year, he also discovered potassium using the same method. Lithium was discovered in 1817 by Johan August Arfwedson, and the remaining elements, cesium and rubidium, were identified using spectroscopy by German chemists in 1860-1861. Francium, the heaviest alkali metal, was discovered in 1939 by French physicist Marguerite Perey.
The unique properties of alkali metals have made them important in various fields such as chemistry, industry, and medicine. They have low melting and boiling points, and are good conductors of heat and electricity, making them useful in batteries, alloys, and as catalysts in chemical reactions. They are also important in medicine for their use in treating bipolar disorder and other mental illnesses. Due to their reactive nature, they require careful handling and storage, and their usage is often regulated.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – alkali metal |
Alkali metals are a group of six highly reactive chemical elements – lithium, sodium, potassium, rubidium, cesium, and francium – located in group 1 of the periodic table.
History

The discovery and use of alkali metals date back to ancient times. Historically, sodium carbonate or soda ash was obtained from the ashes of certain plants grown in sodium-rich soils and has been an important part of human civilization for various applications, such as making soap, glass, and as a flux in metallurgy. Potassium nitrate or saltpetre was used in gunpowder, which was invented in China in the 9th century CE and gunpowder was first brought to Europe in the 1200s.
It was not until the early 19th century that the individual alkali metals were isolated and identified. In 1807, Sir Humphry Davy used electrolysis to isolate potassium and then sodium. He derived the name sodium from the Arabic word “suda,” meaning headache, as it was historically used to treat headaches. On the other hand, the name potassium is derived from the word “potash,” which refers to an early method of extracting various potassium salts.
Lithium was discovered by Johan August Arfwedson in 1817 while analyzing the mineral petalite. The element was not isolated in pure form until 1855 by Robert Bunsen and Augustus Matthiessen. The name lithium comes from the Greek word “lithos” meaning stone. Cesium and rubidium were discovered by Robert Bunsen and Gustav Kirchhoff in 1860 and 1861 while they were studying mineral waters. Cesium is named after the Latin word “caesius” meaning sky-blue, while rubidium is named after the Latin word “rubidus” meaning dark red. Francium, the last element in the alkali metal group, was discovered in 1939 by French physicist Marguerite Perey through the radioactive decay of actinium. It was named after France, the country of its discovery.
The alkali metals were initially used in small quantities for their reactivity, with sodium being used as a reagent in the manufacture of aluminum. With the establishment of the electrolytic process for aluminum purification, the use of sodium was expected to decrease. However, improvements in the electrolytic production of sodium led to a reduction in its cost, making it economically viable for the manufacture of various products. Alkali metals are currently widely used in various industries, including agriculture (fertilizers), energy storage (batteries), healthcare (medicine), and electronics.
Occurrence and production
Alkali metals are found in the earth’s crust, with their abundance ranging from sodium as the most abundant to francium as the least abundant. They are generally rare in nature and occur in small quantities in minerals and mineral waters. Sodium and potassium are the most abundant alkali metals and are found in minerals such as halite (NaCl) and sylvite (KCl). Lithium is found in minerals such as spodumene, petalite, and lepidolite. Rubidium and cesium are found in lepidolite and pollucite. Francium is considered the rarest of all the alkali metals and is only present in trace amounts in uranium and thorium ores.
The production of alkali metals involves the use of various methods depending on the element required. The production of sodium involves the electrolysis of molten sodium chloride, while potassium is produced via the electrolysis of molten potassium chloride. Lithium is produced by electrolyzing a mixture of lithium chloride and potassium chloride at high temperature. Cesium is extracted from pollucite by methods like acid digestion, alkaline decomposition, and direct reduction, while rubidium is generally obtained as a byproduct of lithium extraction from lepidolite. Francium has limited production and it occurs only as a result of the radioactive decay of other elements. Therefore, there are no commercial production methods for francium.
Properties
Physical properties
Alkali metals are the softest metals and can be easily cut with a knife. They have a low density and a low melting point, which makes them good conductors of heat and electricity. They are silvery-white in color and have a shiny surface when freshly cut, but tarnish quickly in air due to their high reactivity.
Chemical properties
Alkali metals have one electron in their outermost shell, which makes them highly reactive and easily lose that electron to form a +1 ion. They are the most reactive group of metals in the periodic table, and react violently with water to produce hydrogen gas and a strong alkaline solution. They also react with nonmetals such as halogens to form salts.
Electronegativity
Alkali metals have low electronegativity, meaning they have a low tendency to attract electrons towards themselves. This makes them highly reactive and more likely to give up electrons than to accept them.
Ionization energy
Alkali metals have low ionization energy, meaning it requires relatively little energy to remove the outermost electron from the atom. This property makes them highly reactive and easily ionized.
Flame test

Alkali metals are known for their characteristic flame color when heated. Each metal gives a unique color to the flame due to the energy absorbed and emitted by electrons during the transition from one energy level to another. For example, potassium gives a violet flame, while sodium gives a yellow flame.
Isotopes
Alkali metals have several isotopes with varying atomic masses, but the most common isotope for each element is usually the most stable. For example, the most common isotopes of lithium are lithium-6 and lithium-7, while the most common isotope of rubidium is rubidium-85.
Trends
Atomic size
The atomic size of alkali metals increases as you go down the group. This is because the number of electron shells increases, and there is less attraction between the nucleus and the outermost electron.
Ionization energy
Alkali metals have a low ionization energy, which means that they readily lose their outermost electron to form a positive ion. The ionization energy decreases as you go down the group due to the increased distance between the nucleus and the outermost electron.
Reactivity
Alkali metals are highly reactive and readily form compounds with other elements. The reactivity increases as you go down the group because the outermost electron is farther from the nucleus and is held less tightly.
Melting and boiling points
The melting and boiling points of alkali metals decrease as you go down the group. This is due to the weak metallic bonding between the atoms, which is further weakened by the increased atomic size.
Density
The density of alkali metals increases as you go down the group. This is because the atoms have more mass and there are more atoms packed into the same volume.
Electronegativity
Alkali metals have a low electronegativity, which means that they tend to lose electrons rather than gain them. The electronegativity decreases as you go down the group due to the increased distance between the nucleus and the outermost electron.
Applications
Industrial
The alkali metals find wide applications in the industry due to their unique properties. Sodium is widely used in the production of organic compounds, dyes, and as a heat exchanger in some nuclear reactors. Potassium is used in fertilizers, as a heat transfer medium, and in the production of soap and glass. Lithium is used in the production of batteries, ceramics, and heat-resistant glass. Rubidium and cesium are used in atomic clocks, while francium is used in scientific research.
Medical and scientific
Alkali metals have numerous medical and scientific applications. Sodium and potassium ions play a vital role in the functioning of the human body, and their concentration needs to be carefully regulated. Lithium is widely used as a mood stabilizer in the treatment of bipolar disorder. Rubidium and cesium isotopes are used in nuclear medicine for diagnostic imaging and cancer treatment.
Daily life and consumer products
Alkali metals are also present in our daily life and consumer products. Sodium and potassium are essential nutrients and are found in various foods. Sodium is also present in table salt and baking soda. Lithium-ion batteries, which are used in smartphones, laptops, and electric vehicles, contain lithium. Rubidium and cesium are used in GPS systems and atomic clocks, which are found in smartphones and other devices.
Interesting facts
Alkali metals are so reactive that they can explode when exposed to water or air.

Lithium is the lightest metal and can float on both water and oil.
Sodium is the most common alkali metal and is essential for life as it helps in regulating body fluids.
Potassium is important for muscle function and maintaining heart rhythm.
Rubidium is used in atomic clocks, which are the most accurate timekeeping devices available.
Cesium is the most electropositive element, meaning it has the highest tendency to donate electrons, and is used in photoelectric cells and atomic clocks.
Francium is the least stable of all the naturally occurring elements and has a half-life of only 22 minutes.
Alkali metals can be used as catalysts in some chemical reactions.
Alkali metals have a low melting point and are very malleable, making them useful in various applications.
The alkali metals are so reactive that they can ignite spontaneously in air at room temperature.
Alkali metals can be used in the production of alloys with other metals.
Alkali metals have a unique ability to form alloys with other metals which can enhance properties like strength, corrosion resistance, and conductivity.
Rubidium and cesium have been used in research to create Bose-Einstein condensates, a state of matter in which particles act like waves.
Alkali metals such as sodium and potassium are used in the manufacturing of soaps and detergents due to their ability to react with fatty acids and produce salts known as soaps.
Some alkali metals have been used in nuclear medicine for imaging and treatment purposes.
Related
More topics
- Block (periodic table)
- Group (periodic table)
- Period (periodic table)
- Metal
- Alkali metal
- Alkaline earth metal
- Lanthanide
- Actinide
- Transition metal
- Post-transition metal
- Metalloid
- Nonmetal
- Reactive nonmetal
- Noble gas
External links
- Alkali metal | Definition, Properties, & Facts – Britannica
- Alkali metal – Wikipedia
- Group 1A — The Alkali Metals – Angelo State University
- Information on Alkali Metals – Stanford Environmental Health & Safety
- The alkali metals: 200 years of surprises – The Royal Society
- Alkali Metals – Periodic Table – ChemTalk
- Group 1: Hydrogen and the Alkali Metals – Chemistry LibreTexts
- Alkali Metals – Chemical Elements.com
- Alkali Metals: Elements in the First Column of the Periodic Table – HowStuffWorks
- Alkali Metals – School City of Hobart
- Alkali Metals – Diamond Light Source
- Alkali Metals – Purdue University
- Alkali Metals | Definition, Properties & Characteristics – Study.com
- The Reactivity of Alkali Metals Explained (animation) – Annenberg Learner
- Elements & Periodic Table: Alkali Metals – Chem4Kids
- Alkali Metal – an overview – ScienceDirect
- Physical properties of the alkali metals – Group 1 – BBC
- The Alkali Metals: Part 1 | Resource – RSC Education
- Lesson Explainer: Alkali Metals – Nagwa Limited
- Alkali Metals – The Periodic Table Classification Of Elements Into Groups By Electronic Structure – Jack Westin
- Group 1: The Alkali Metals – Breaking Atom
- Chemistry for Kids: Elements – Alkali Metals – Ducksters
- Alkali Metal Definition (Chemistry) – ThoughtCo
- Periodic Table of the Elements – Alkali Metals – Cool Periodic Table
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.





