
Caesium (Cs), also spelled as cesium in American English, is a chemical element of the periodic table, located in group 1 and period 6, and has the atomic number 55. It is a soft, silvery-gold alkali metal whose name comes from the Latin word “caesius” (which means sky blue). In 1860, German chemist Robert Bunsen and physicist Gustav Kirchhoff discovered caesium using the newly invented method of flame spectroscopy.[1] It is a relatively rare element, with a total abundance in the Earth’s crust of about 3 parts per million.
Caesium reacts spontaneously in the air, which makes it hard to handle. This highly reactive silvery metal melts just above room temperature at 28.5 ℃ (83.3 ℉). Due to its reactive nature, caesium is considered a hazardous material and must be stored and transported separately for safety reasons. With a Pauling scale value of 0.79, it is the least electronegative element. It is one of the elements with the most isotopes, with 40 known isotopes ranging from Caesium-112 to Caesium-151.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – s block |
Caesium is an s-block element, found in the first column and the sixth row of the periodic table. It has the atomic number 55 and is denoted by the symbol Cs.
Element information
 |
|
 |
|
| Origin of name | Latin word “caesius” (which means sky blue) |
| Symbol | Cs |
| Atomic number (Z) | 55 |
| Atomic mass | 132.90545 u |
| Block | s-block |
| Group | 1 |
| Period | 6 |
| Classification | Alkali metal |
| Atomic radius | 265 pm |
| Covalent radius | 244±11 pm |
| Van der Waals radius | 343 pm |
| Melting point | 28.5 ℃, 83.3 ℉, 301.7 K |
| Boiling point | 671 ℃, 1240 ℉, 944 K |
| Electron configuration | [Xe] 6s1 |
| Electrons per shell | 2, 8, 18, 18, 8, 1 |
| Learn how to draw: Caesium Bohr model | |
| Crystal structure | Body-centered cubic (bcc) |
| Phase at r.t | Solid |
| Density near r.t | 1.93 g/cm3 |
| Main isotopes | Caesium-133 |
| Natural occurrence | Primordial |
| Oxidation state | +1 |
| Electronegativity (Pauling scale) | 0.79 |
| Protons Neutrons Electrons |
55 78 55 |
| Valence electrons | 1 |
| Learn how to find: Caesium valence electrons | |
| CAS number | 7440-46-2 |
| Discovered by | Robert Bunsen and Gustav Kirchhoff in 1860 |
History
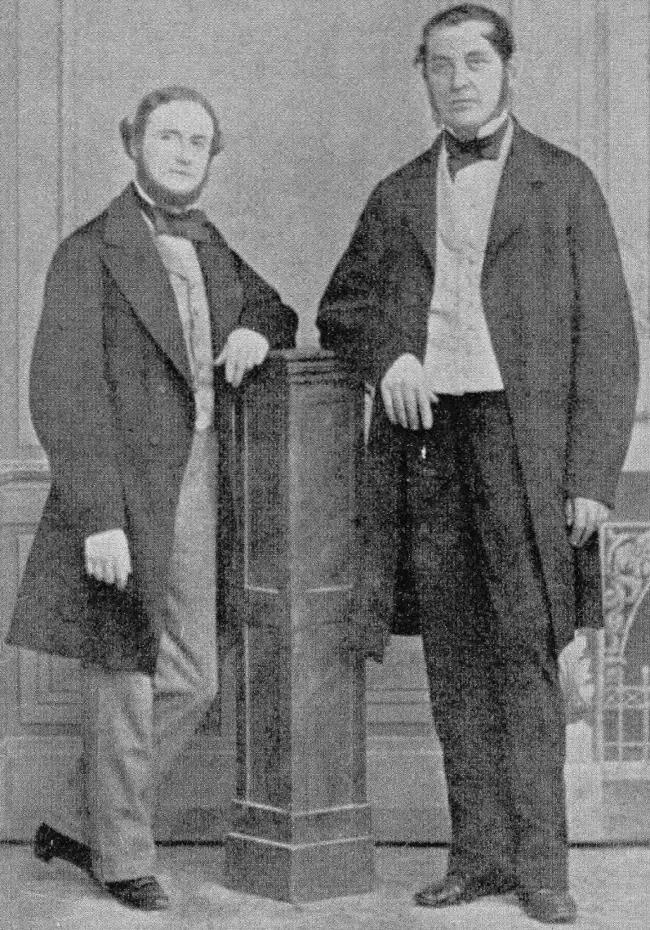
In 1860, Robert W. Bunsen and Gustav R. Kirchhoff were the first to discover the element caesium through spectroscopic means, using the spectroscope they had just invented the year before. They attempted to create elemental caesium through the electrolysis of molten caesium chloride, but instead obtained a blue, homogeneous substance. It was not until later, when German chemist Carl Setterberg at the University of Bonn electrolyzed caesium cyanide CsCN, that pure caesium metal was successfully isolated.
Caesium has traditionally been used mostly in research and development. Before the 1920s, it was primarily used in vacuum tubes as a “getter” to remove trace amounts of oxygen and other gasses that became trapped in the tube when it was sealed. Prior to this time, the element had no significant practical applications.[2]
Occurrence
Caesium is found in small amounts, around 3 parts per million, in the Earth’s crust in the minerals pollucite, rhodizite, and lepidolite. It is more abundant than a few well-known metals like tungsten, antimony, and cadmium. Despite being chemically similar to rubidium, it is 30 times less abundant than rubidium. Only a few minerals contain considerable amounts of caesium. These include several unusual types of beryl, avogadrite, and rhodizite.
Pollucite is the only economically significant source mineral for caesium.[2] The Tanco Mine in Manitoba, Canada is the leading and most abundant source of caesium in the world, with an estimated 350,000 metric tons of pollucite ore, representing over two-thirds of the global pollucite reserves. Large pollucite deposits have also been discovered in Zimbabwe, as well as in lithium-bearing pegmatites in Bernic Lake in Manitoba, Canada.
Production
Because caesium is naturally found combined with other alkali metals and always found with rubidium, producing pure caesium has a number of challenges. Caesium has mostly been produced from its ore pollucite, using three methods: acid digestion, alkaline decomposition, and direct reduction. Also, caesium metal can be produced from the ore’s purified compounds. Cesium chloride and other cesium halides can be reduced to caesium metal using calcium or barium at a temperature of 700 ℃ to 800 ℃. The resulting caesium metal can then be distilled and collected.
Properties
Caesium is a metal that is known for its soft and ductile nature, as well as its low melting point of 28.5 ℃. Due to its extremely low melting point, it often exists as a liquid that looks similar to mercury. Actually, it is the only metal that has a melting point lower than caesium.[3]
Caesium is also highly reactive, making it the most electropositive and reactive of the alkali metals. It has a tendency to ignite spontaneously when exposed to air and reacts explosively when it comes into contact with water.
Due to its reactive nature, caesium, like rubidium, is typically stored and transported in dry mineral oil, dry saturated hydrocarbons, or in an inert atmosphere or vacuum sealed in a borosilicate glass ampoule. This helps to prevent any unintended reactions with the surrounding environment.
Applications
Since the 1990s, the most often utilized form of caesium is found in cesium formate brines, a high-density, low-viscosity fluid used in high-temperature, high-pressure oil and gas drilling.[4]
Caesium is used in atomic clocks. The element also plays a crucial role in GPS, cell phone transmissions, the Internet, and aircraft guidance systems.
Caesium is utilized in photoelectric cells, which turn sunlight into energy. Direct sunlight stimulates the electrons in caesium atoms, and in photoelectric cells, these electrons flow to produce an electric current.
Caesium-137 can be used to enhance specific qualities of different food products by sterilization, killing insect pests and bacteria without noticeably changing the temperature of the food.
Caesium is added as a carbonate in optical glass to improve stability and durability. Similar to rubidium, caesium has the potential to be used as an ion engine fuel.
Additionally, caesium is used as a getter in vacuum tubes, in cancer treatment, in lasers and fluorescent lamps, and its compounds are used as an oxidizer in infrared flares.
Interesting facts
Caesium was discovered using a flame emission spectroscopy method.
Caesium has a very low melting point that if you place it on your hand, it will melt.
Caesium reacts explosively with oxygen and water, so it is often stored in mineral oil or kerosene.
Along with francium, caesium is also the most reactive metal.
Caesium has only one stable isotope, 133Cs.
Caesium is the 47th most abundant element on earth.
Caesium is the most electropositive chemical element.
Related
More elements
References
1. C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – CESIUM
3. Exploring chemical elements and their compounds
4. Mineral Resource of the Month: Cesium – EARTH Magazine
External links
- https://www.rsc.org/periodic-table/element/55/caesium
- https://en.wikipedia.org/wiki/Caesium
- https://www.britannica.com/science/cesium
- https://pubchem.ncbi.nlm.nih.gov/element/Cesium
- https://www.chemicool.com/elements/cesium.html
- https://www.livescience.com/37578-cesium.html
- https://chemistrytalk.org/cesium-element/
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
