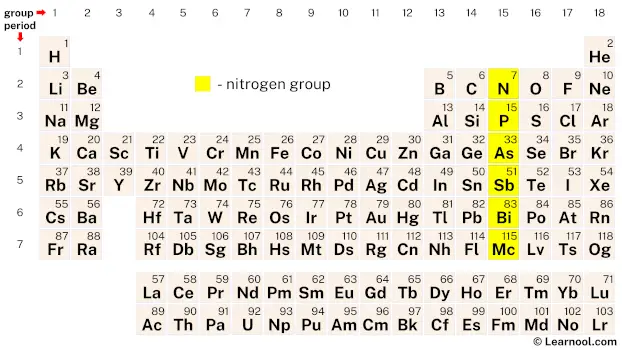
The nitrogen group, also referred to as the nitrogen family, pentals, pnictogens, or group Ⅴ, consists of six chemical elements in the periodic table: nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), bismuth (Bi), and moscovium (Mc). These elements, located in group 15, share similar characteristics due to their common electron configuration of five valence electrons. Nitrogen, the lightest element in the group, is a reactive nonmetal, as is phosphorus. Arsenic and antimony, known as metalloids, exhibit properties of both metals and nonmetals. Bismuth, on the other hand, is categorized as a post-transition metal. The recently discovered element moscovium is a synthetic element and completes the nitrogen group.
The elements of the nitrogen group are located in the p-block of the periodic table. This block represents the elements with their outermost electrons in the p-orbital. The applications of these elements vary widely. Phosphorus finds extensive use in fertilizers, where it plays a crucial role in promoting plant growth and development. It is also utilized in the production of various chemicals, such as detergents and flame retardants. Arsenic, although highly toxic, has applications in certain industries, including the production of semiconductors and wood preservatives. These diverse applications highlight the significance of elements from the nitrogen group in various fields of science, technology, and industry.
On periodic table
| group | ⇨ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| period | ⇩ | ||||||||||||||||||
| 1 | 1 H  Hydrogen |
2 He  Helium |
|||||||||||||||||
| 2 | 3 Li  Lithium |
4 Be  Beryllium |
5 B  Boron |
6 C  Carbon |
7 N  Nitrogen |
8 O  Oxygen |
9 F  Fluorine |
10 Ne  Neon |
|||||||||||
| 3 | 11 Na  Sodium |
12 Mg  Magnesium |
13 Al  Aluminium |
14 Si Silicon |
15 P  Phosphorus |
16 S  Sulfur |
17 Cl  Chlorine |
18 Ar  Argon |
|||||||||||
| 4 | 19 K  Potassium |
20 Ca  Calcium |
21 Sc  Scandium |
22 Ti  Titanium |
23 V  Vanadium |
24 Cr  Chromium |
25 Mn  Manganese |
26 Fe  Iron |
27 Co  Cobalt |
28 Ni  Nickel |
29 Cu  Copper |
30 Zn  Zinc |
31 Ga  Gallium |
32 Ge  Germanium |
33 As  Arsenic |
34 Se  Selenium |
35 Br  Bromine |
36 Kr  Krypton |
|
| 5 | 37 Rb  Rubidium |
38 Sr  Strontium |
39 Y  Yttrium |
40 Zr  Zirconium |
41 Nb  Niobium |
42 Mo  Molybdenum |
43 Tc  Technetium |
44 Ru  Ruthenium |
45 Rh  Rhodium |
46 Pd  Palladium |
47 Ag  Silver |
48 Cd  Cadmium |
49 In  Indium |
50 Sn  Tin |
51 Sb  Antimony |
52 Te  Tellurium |
53 I  Iodine |
54 Xe  Xenon |
|
| 6 | 55 Cs  Caesium |
56 Ba  Barium |
72 Hf  Hafnium |
73 Ta  Tantalum |
74 W  Tungsten |
75 Re  Rhenium |
76 Os  Osmium |
77 Ir  Iridium |
78 Pt  Platinum |
79 Au  Gold |
80 Hg  Mercury |
81 Tl  Thallium |
82 Pb  Lead |
83 Bi  Bismuth |
84 Po  Polonium |
85 At  Astatine |
86 Rn  Radon |
||
| 7 | 87 Fr  Francium |
88 Ra  Radium |
104 Rf  Rutherfordium |
105 Db  Dubnium |
106 Sg  Seaborgium |
107 Bh  Bohrium |
108 Hs  Hassium |
109 Mt  Meitnerium |
110 Ds  Darmstadtium |
111 Rg  Roentgenium |
112 Cn  Copernicium |
113 Nh  Nihonium |
114 Fl  Flerovium |
115 Mc  Moscovium |
116 Lv  Livermorium |
117 Ts  Tennessine |
118 Og  Oganesson |
||
| 57 La  Lanthanum |
58 Ce  Cerium |
59 Pr  Praseodymium |
60 Nd  Neodymium |
61 Pm  Promethium |
62 Sm  Samarium |
63 Eu  Europium |
64 Gd  Gadolinium |
65 Tb  Terbium |
66 Dy  Dysprosium |
67 Ho  Holmium |
68 Er  Erbium |
69 Tm  Thulium |
70 Yb  Ytterbium |
71 Lu  Lutetium |
|||||
| 89 Ac  Actinium |
90 Th  Thorium |
91 Pa  Protactinium |
92 U  Uranium |
93 Np  Neptunium |
94 Pu  Plutonium |
95 Am  Americium |
96 Cm  Curium |
97 Bk  Berkelium |
98 Cf  Californium |
99 Es  Einsteinium |
100 Fm  Fermium |
101 Md  Mendelevium |
102 No  Nobelium |
103 Lr  Lawrencium |
|||||
| – nitrogen group |
On the periodic table, the nitrogen group, also known as group 15, includes six chemical elements – nitrogen, phosphorus, arsenic, antimony, bismuth, and moscovium.
Related
More topics
- Boron group
- Carbon group
- Nitrogen group
- Oxygen group
- Halogen
- Noble gas
External links
- Nitrogen group element | Properties, Uses, & List – Britannica
- Pnictogen – Wikipedia
- Group 15: The Nitrogen Family – Chemistry LibreTexts
- Element Group 15 – Nitrogen Family Facts – ThoughtCo
- Nitrogen – Element information, properties and uses – Royal Society of Chemistry
- Group 5A — The Pnictogens – Angelo State University
- The Nitrogen Group of Elements – HyperPhysics Concepts
- Nitrogen Family – Encyclopedia.com
- Carbon and Nitrogen Groups – AK Lectures
- Nitrogen Group – an overview – ScienceDirect
- Nitrogen family Definition & Meaning – Merriam-Webster
- Group 15 The Pnictogen (Nitrogen Family) – Kent’s Chemistry
- Nitrogen group – wikidoc
- Boron Family – Carbon Family – Nitrogen Family – Concept – Brightstorm
- Nitrogen group – Scientific Library
- Group 15 elements – Bartleby
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.





