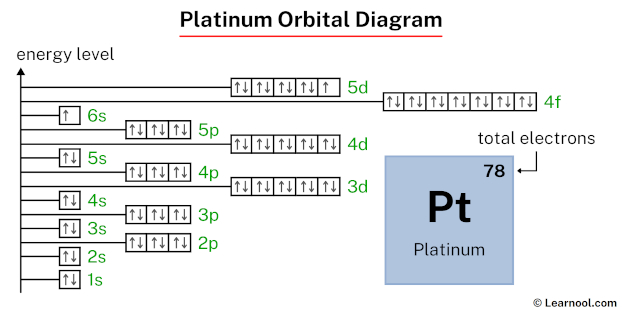
In the platinum orbital diagram, the 1s subshell holds two electrons, the 2s subshell carries another pair, the 2p subshell encompasses six electrons, the 3s subshell contains two electrons, the 3p subshell carries six electrons, the 4s subshell holds two electrons, the 3d subshell carries ten electrons, the 4p subshell accommodates six electrons, the 5s subshell has two electrons, the 4d subshell carries ten electrons, the 5p subshell accommodates six electrons, the 6s subshell has one electron, the 4f subshell carries fourteen electrons, and the 5d subshell has nine electrons, totaling seventy-eight electrons.
To illustrate the platinum orbital diagram, begin by determining the number of electrons from the periodic table. Take note of the electron configuration for reference and follow the three fundamental rules: the Aufbau principle, Pauli exclusion principle, and Hund’s rule. This systematic approach ensures an accurate representation of platinum’s orbital arrangement.
Steps
Find electrons
The atomic number of platinum represents the total number of electrons of platinum. Since the atomic number of platinum is 78, the total electrons of platinum are 78.
Write electron configuration
The electron configuration of platinum is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s1 4f14 5d9.
Now in the next step, start drawing the orbital diagram for platinum.
Draw orbital diagram
Before drawing the orbital diagram, you should know the three general rules.
- Aufbau principle – electrons are first filled in lowest energy orbital and then in higher energy orbital
- Pauli exclusion principle – two electrons with the same spin can not occupy the same orbital
- Hund’s rule – each orbital should be first filled with one electron before being paired with a second electron
Also, you should know the number of orbitals in each subshell.
We can calculate the number of orbitals in each subshell using the formula: 2ℓ + 1
Where, ℓ = azimuthal quantum number of the subshell
For s subshell, ℓ = 0
For p subshell, ℓ = 1
For d subshell, ℓ = 2
For f subshell, ℓ = 3
So each s subshell has one orbital, each p subshell has three orbitals, each d subshell has five orbitals, and each f subshell has seven orbitals.
Now start to draw!
As mentioned above, the electron configuration of platinum is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s1 4f14 5d9. Hence, draw the blank orbital diagram of platinum up to 5d subshell as follows:
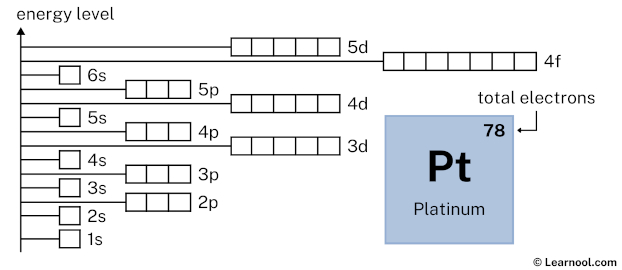
In the above orbital diagram, the box represents an orbital. Each orbital has a capacity of two electrons. And the arrows (↑↓) are drawn inside the box to represent electrons.
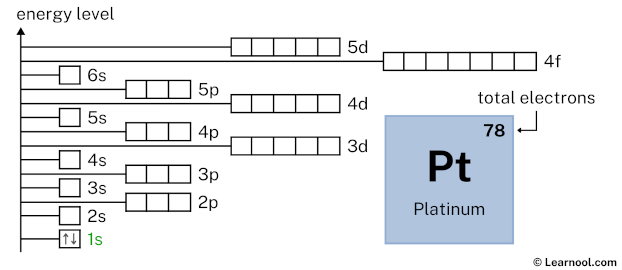
Now 1s2 indicates that the 1s subshell has 2 electrons. So draw two arrows in the 1s box showing two electrons as follows:
2s2 indicates that the 2s subshell has 2 electrons. So draw two arrows in the 2s box showing two electrons as follows:
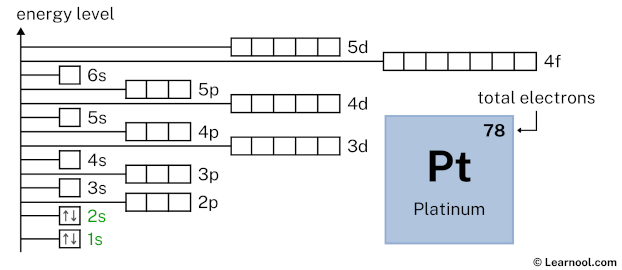
2p6 indicates that the 2p subshell has 6 electrons. So draw six arrows in the 2p box showing six electrons as follows:
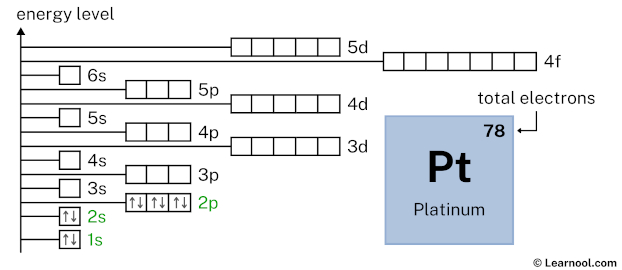
3s2 indicates that the 3s subshell has 2 electrons. So draw two arrows in the 3s box showing two electrons as follows:
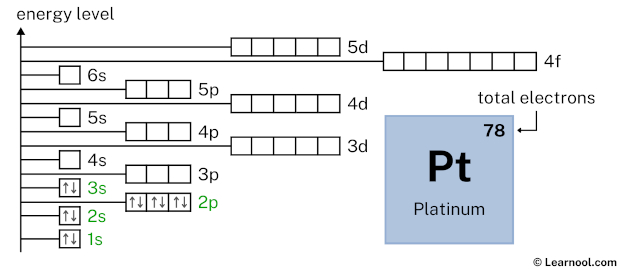
3p6 indicates that the 3p subshell has 6 electrons. So draw six arrows in the 3p box showing six electrons as follows:
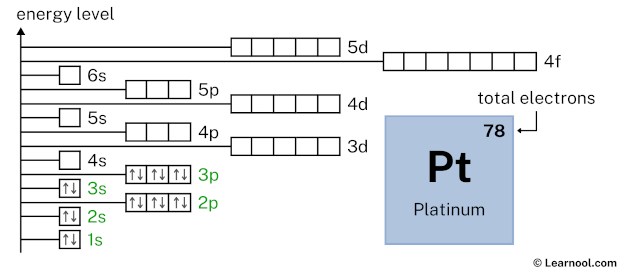
4s2 indicates that the 4s subshell has 2 electrons. So draw two arrows in the 4s box showing two electrons as follows:
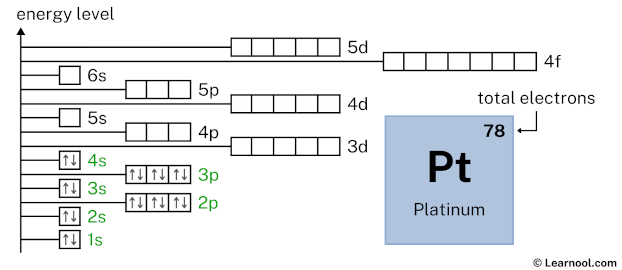
3d10 indicates that the 3d subshell has 10 electrons. So draw ten arrows in the 3d box showing ten electrons as follows:
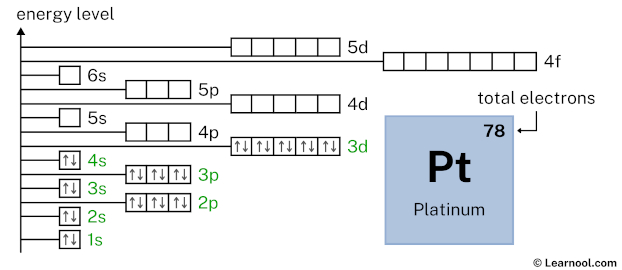
4p6 indicates that the 4p subshell has 6 electrons. So draw six arrows in the 4p box showing six electrons as follows:
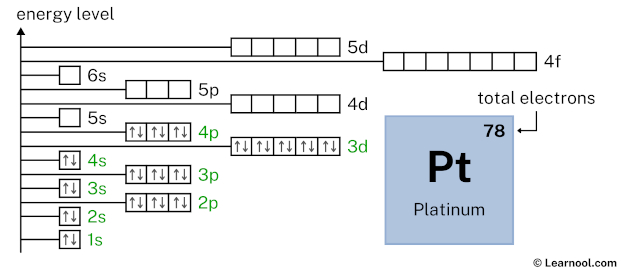
5s2 indicates that the 5s subshell has 2 electrons. So draw two arrows in the 5s box showing two electrons as follows:
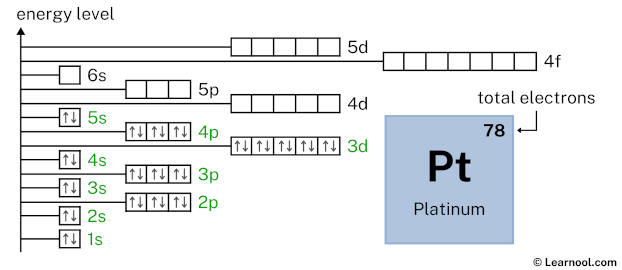
4d10 indicates that the 4d subshell has 10 electrons. So draw ten arrows in the 4d box showing ten electrons as follows:
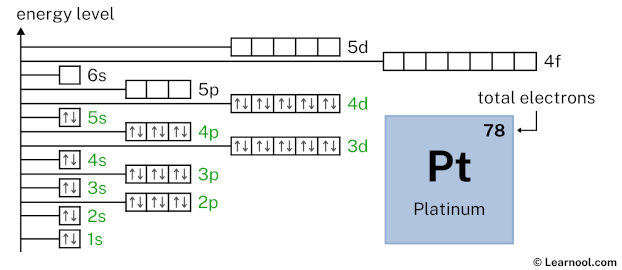
5p6 indicates that the 5p subshell has 6 electrons. So draw six arrows in the 5p box showing six electrons as follows:
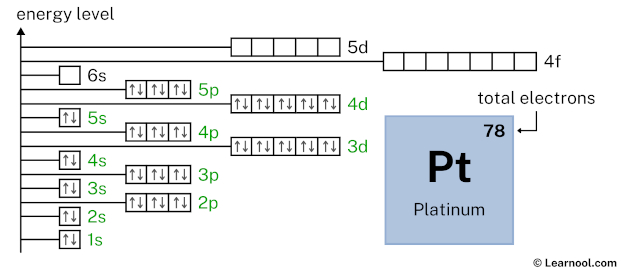
6s1 indicates that the 6s subshell has 1 electron. So draw one arrow in the 6s box showing one electron as follows:
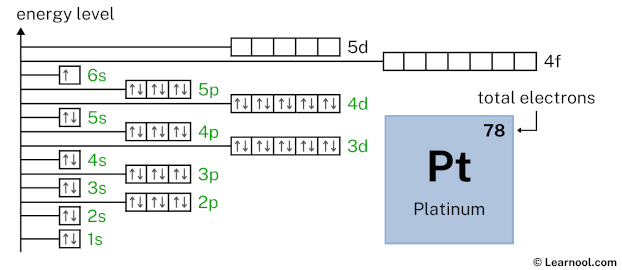
4f14 indicates that the 4f subshell has 14 electrons. So draw fourteen arrows in the 4f box showing fourteen electrons as follows:
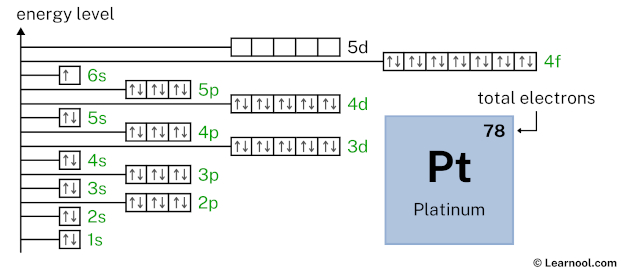
5d9 indicates that the 5d subshell has 9 electrons. So draw nine arrows in the 5d box showing nine electrons as follows:
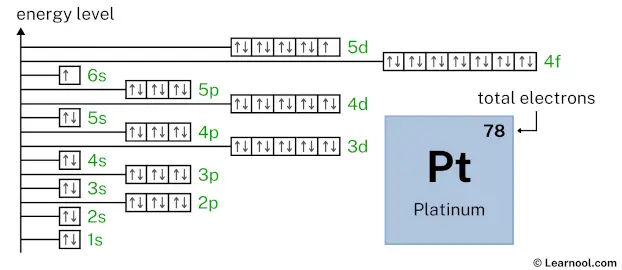
That’s it! This is the final orbital diagram of platinum as we have used all 78 electrons.
Question 1: Why does the 6s subshell have one electron (instead of two electrons), and the 5d subshell has nine electrons (instead of eight electrons)?
Answer: One partially filled 5d subshell is more stable than one empty 5d subshell. That’s why the 5d subshell has nine electrons (instead of eight electrons). And the 6s subshell has one electron (instead of two electrons).
Question 2: What if the 6s subshell has zero electrons (instead of one electron), and the 5d subshell has ten electrons (instead of nine electrons)?
Answer: A partially filled 6s subshell is more stable than an empty 6s subshell. That’s why the 6s subshell has one electron (instead of zero electrons). And the 5d subshell has nine electrons (instead of ten electrons).
Next: Neodymium orbital diagram
Related
More topics
External links
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
