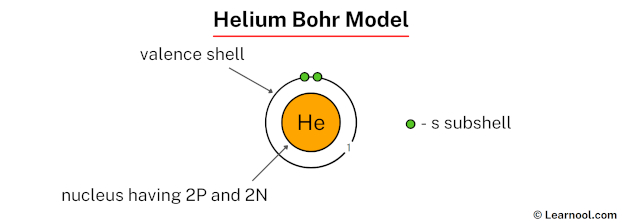
The Bohr model of helium depicts a central nucleus composed of 2 protons and 2 neutrons.[1][2][3] These subatomic particles are tightly packed at the center of the atom, forming its dense core. Surrounding the nucleus are electron shells arranged in circular paths. Since helium has only 2 electrons, both are located in the first shell, known as the K shell. This shell can hold a maximum of 2 electrons, so it is completely filled in the helium’s case, and no additional shells are required.
To draw the Bohr model of helium, begin by illustrating the nucleus at the center and indicating the presence of 2 protons and 2 neutrons inside it. Next, draw a single circular shell around the nucleus to represent the K shell. Place both electrons on this shell, spaced apart to show their positions in orbit. As helium has no more than 2 electrons, the model is complete with just one electron shell surrounding the nucleus.
Steps
Write protons, neutrons, and electrons of helium atom
Helium has 2 protons, 2 neutrons, and 2 electrons.
Learn how to find: Helium protons neutrons electrons
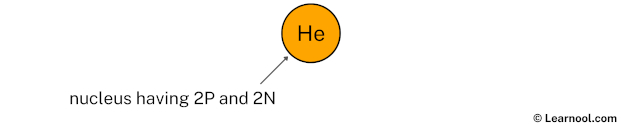
Draw nucleus of helium atom
The nucleus of a helium atom contains 2 protons and 2 neutrons. So draw the nucleus of helium atom as follows:
Now in the next step, draw the 1st electron shell and start marking electrons.
Draw 1st electron shell
Remember that we have a total of 2 electrons.
The 1st electron shell (containing s subshell) can hold up to a maximum of 2 electrons. So draw the 1st electron shell as follows:
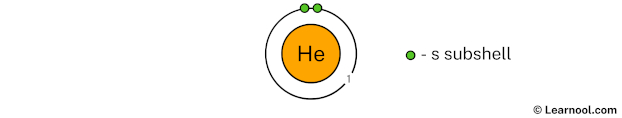
In the above image, 1 represents the 1st electron shell that contains 1s subshell. And the green color represents the number of electrons in that subshell. This means that the 1st electron shell has a total of 2 electrons.
That’s it! This is the final Bohr model of helium atom as we have only 2 electrons and that are marked in the 1st electron shell.
Next: Lithium Bohr model
Related
More topics
References
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
