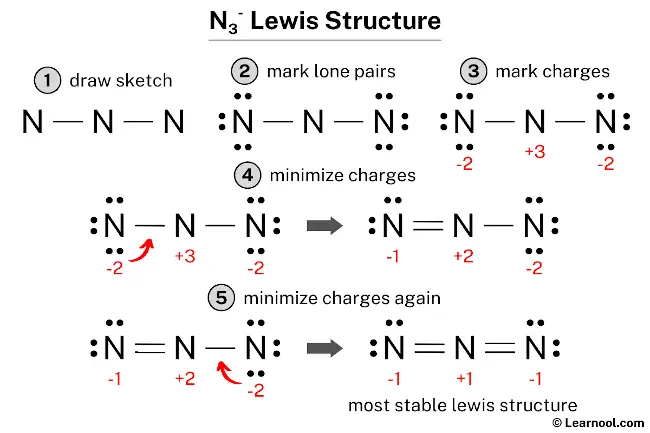
N3– (azide) has three nitrogen atoms.
In the N3– Lewis structure, there are two double bonds around the nitrogen atom, with two other nitrogen atoms attached to it, and on the left and right nitrogen atoms, there are two lone pairs.
Also, there is a negative (-1) charge on the left and right nitrogen atoms, and a positive (+1) charge on the center nitrogen atom.
Alternative method: Lewis structure of N3–
Steps
To properly draw the N3– Lewis structure, follow these steps:
#1 Draw a rough sketch of the structure
#2 Next, indicate lone pairs on the atoms
#3 Indicate formal charges on the atoms, if necessary
#4 Minimize formal charges by converting lone pairs of the atoms
#5 Repeat step 4 if necessary, until all charges are minimized
Let’s break down each step in more detail.
#1 Draw a rough sketch of the structure
- First, determine the total number of valence electrons
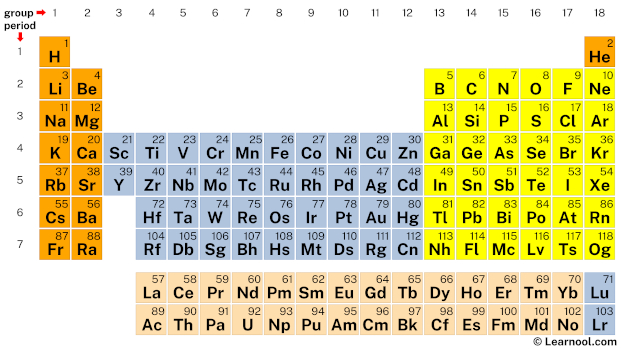
In the periodic table, nitrogen lies in group 15. Hence, nitrogen has five valence electrons.
Since N3– has three nitrogen atoms, so…
Valence electrons of three nitrogen atoms = 5 × 3 = 15
Now the N3– has a negative (-1) charge, so we have to add one more electron.
So the total valence electrons = 15 + 1 = 16
Learn how to find: Nitrogen valence electrons
- Second, find the total electron pairs
We have a total of 16 valence electrons. And when we divide this value by two, we get the value of total electron pairs.
Total electron pairs = total valence electrons ÷ 2
So the total electron pairs = 16 ÷ 2 = 8
- Third, determine the central atom
Here, there are three atoms and all atoms are nitrogen, so we can assume any one as the central atom.
Let’s assume that the central atom is center nitrogen.
- And finally, draw the rough sketch
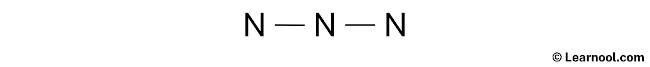
#2 Next, indicate lone pairs on the atoms
Here, we have a total of 8 electron pairs. And two N — N bonds are already marked. So we have to only mark the remaining six electron pairs as lone pairs on the sketch.
Also remember that nitrogen is a period 2 element, so it can not keep more than 8 electrons in its last shell.
Always start to mark the lone pairs from outside atoms. Here, the outside atoms are left nitrogen and right nitrogen.
So for left nitrogen and right nitrogen, there are three lone pairs, and for canter nitrogen, there is zero lone pair because all six electron pairs are over.
Mark the lone pairs on the sketch as follows:
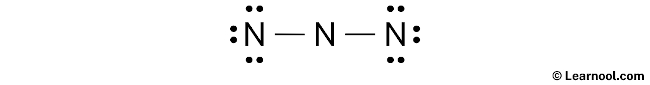
#3 Indicate formal charges on the atoms, if necessary
Use the following formula to calculate the formal charges on atoms:
Formal charge = valence electrons – nonbonding electrons – ½ bonding electrons
For left nitrogen and right nitrogen atom, formal charge = 5 – 6 – ½ (2) = -2
For center nitrogen atom, formal charge = 5 – 0 – ½ (4) = +3
Here, all nitrogen atoms have charges, so mark them on the sketch as follows:
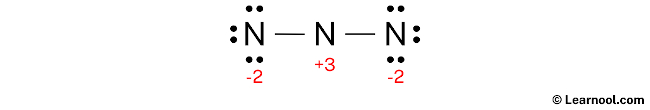
The above structure is not a stable Lewis structure because all nitrogen atoms have charges. Therefore, reduce the charges (as below) by converting lone pairs to bonds.
#4 Minimize formal charges by converting lone pairs of the atoms
Convert a lone pair of the left nitrogen atom to make a new N — N bond with the center nitrogen atom as follows:
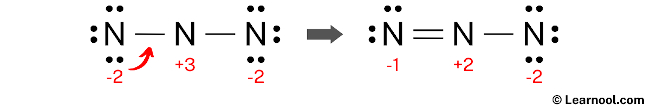
#5 Repeat step 4 (minimize charges again)
Since there are charges on nitrogen atoms, again convert a lone pair of the right nitrogen atom to make a new N — N bond with the center nitrogen atom as follows:
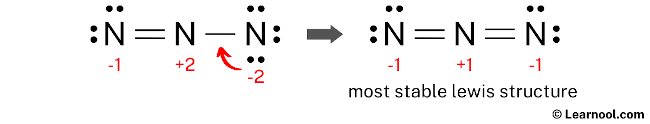
In the above structure, you can see that the central atom (center nitrogen) forms an octet. And the outside atoms (left nitrogen and right nitrogen) also form an octet. Hence, the octet rule is satisfied.
Now there are still charges on the atoms.
This is okay, because the structure with a negative charge on the most electronegative atom is the best Lewis structure. And in this case, the most electronegative element is nitrogen.
Therefore, this structure is the most stable Lewis structure of N3–.
And since the N3– has a negative (-1) charge, mention that charge on the Lewis structure by drawing brackets as follows:
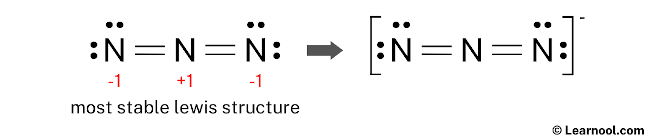
Next: BH3 Lewis structure
External video
- N3- Lewis Structure: How to Draw the Lewis Structure for N3- – YouTube • Wayne Breslyn
External links
- https://lambdageeks.com/n3-lewis-structure/
- https://www.chemistryscl.com/general/N3–lewis-structure/
- https://geometryofmolecules.com/n3-lewis-structure-hybridization-molecular-structure-bond-angle-and-shape/
- https://www.thegeoexchange.org/chemistry/bonding/Lewis-Structures/N3–lewis-structure.html
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
