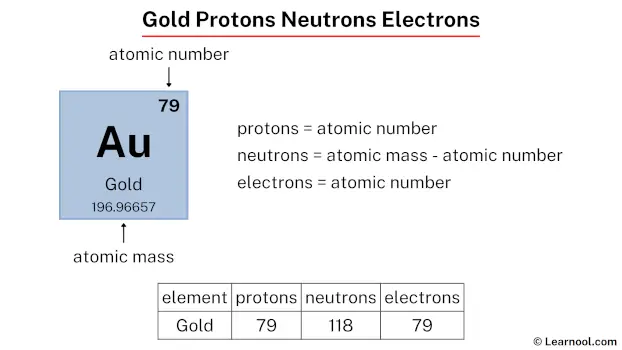
Gold, renowned for its dense, soft, and yellow appearance, is highly valued for its beauty and rarity. It consists of 79 protons, 118 neutrons, and 79 electrons.
Gold protons
- Protons = atomic number
From the periodic table, find the atomic number of gold.
The atomic number of gold is 79. Hence, gold has a total of 79 protons.
Gold neutrons
- Neutrons = atomic mass – atomic number
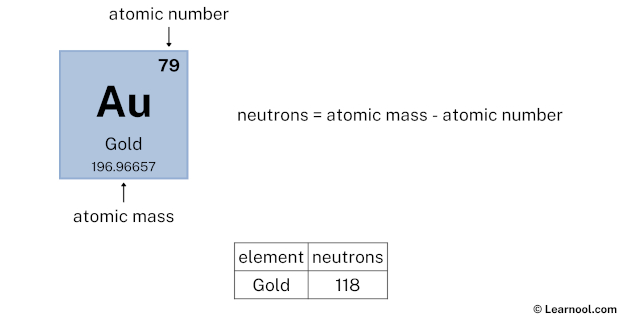
The atomic mass of gold is 196.96657, so we’ll take the roundup value as 197. And the atomic number of gold is 79.
Subtract the atomic number (79) from the atomic mass (197). Hence, gold has a total of 197 – 79 = 118 neutrons.
Gold electrons
- Electrons = atomic number
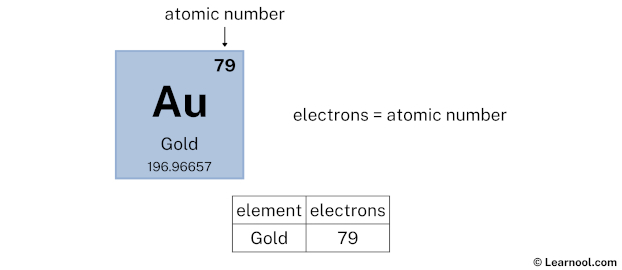
The atomic number of gold is 79. Hence, gold has a total of 79 electrons.
Next: Mercury protons neutrons electrons
Related
More topics
External links
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
