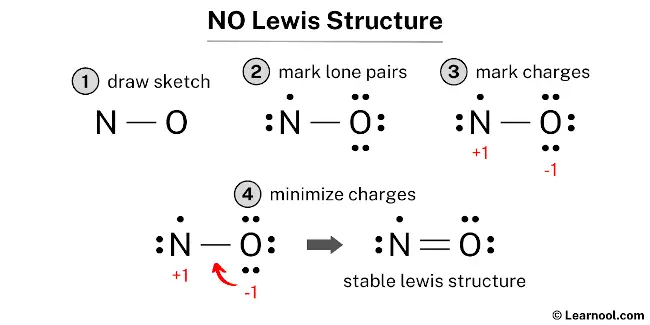
The NO Lewis structure represents the molecular arrangement of nitric oxide, which is composed of one nitrogen atom and one oxygen atom. The structure shows a double bond between the nitrogen and oxygen atoms, with the nitrogen atom having one lone pair and one unpaired electron, and the oxygen atom having two lone pairs.
To draw the NO Lewis structure, begin by sketching a rough skeleton structure of the molecule. Once the rough structure is outlined, identify any lone pairs of electrons on the atoms and represent them in the structure. Next, calculate the formal charges for each atom and minimize them by adjusting lone pairs to form double or triple bonds if necessary, aiming to achieve a stable Lewis structure with reduced charges. If required, repeat the process until all charges are minimized, ensuring that the octet rule is satisfied for both the nitrogen and oxygen atoms. By following these steps, it is possible to accurately draw the NO Lewis structure.
Alternative method: Lewis structure of NO
Rough sketch
The first step in drawing the NO Lewis structure is to determine the total number of valence electrons. The total valence electrons in the molecule can be calculated by multiplying the valence electrons of each atom. NO consists of one nitrogen atom and one oxygen atom. Nitrogen, which belongs to group 15 in the periodic table, has five valence electrons, while oxygen, a group 16 element, has six valence electrons. For the NO molecule, the valence electrons for one nitrogen atom would be 5, and the valence electrons for one oxygen atom would be 6, resulting in a total of 5 + 6 = 11 valence electrons.
Learn how to find: Nitrogen valence electrons and Oxygen valence electrons
After determining the total number of valence electrons, the next step in sketching the NO Lewis structure is to find the total number of electron pairs. To accomplish this, divide the total number of valence electrons by two. In the case of NO, there are 11 valence electrons, which cannot be evenly divided by two. As a result, there are 5 electron pairs and one unpaired electron that need to be marked on the sketch.
The subsequent step in the process involves identifying the central atom, which is chosen based on its lower electronegativity relative to the other atoms in the molecule. In the case of the NO molecule, nitrogen exhibits lower electronegativity than oxygen. Hence, nitrogen is assumed to be the central atom. With this consideration, the rough sketch for the NO Lewis structure can now be drawn.
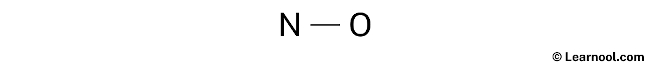
Lone pair
After sketching the rough structure, the next step in drawing the NO Lewis structure is to indicate the lone pairs. In total, there are five electron pairs and one unpaired electron. Since one N – O bond is already marked in the sketch, it means one electron pair is used. Therefore, the remaining four electron pairs and one unpaired electron should be marked as lone pairs on the sketch.
It is important to note that both nitrogen and oxygen belong to period 2 on the periodic table. As a result, they are limited to a maximum of 8 electrons in their outer shell.
When indicating lone pairs, it is important to begin with the outermost atoms. In the case of this molecule, oxygen is the outer atom, so it will have three lone pairs assigned to it. The remaining lone pair and unpaired electron will be assigned to nitrogen. Mark the appropriate lone pairs on the sketch accordingly, following this approach.
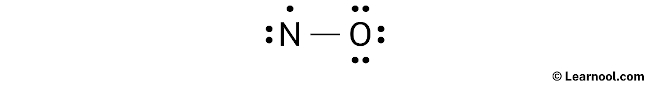
Formal charge
To calculate the formal charges on the atoms in NO, use the formula: Formal charge = valence electrons – nonbonding electrons – ½ bonding electrons. For the nitrogen atom, the formal charge is determined as 5 – 3 – ½ (2) = +1. Similarly, for the oxygen atom, the formal charge is calculated as 6 – 6 – ½ (2) = -1.
Once the formal charges have been calculated for both nitrogen and oxygen atoms, it is crucial to denote these charges on the sketch for accurate representation.
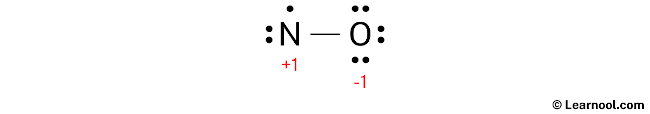
The existing Lewis structure is considered unstable due to the presence of charges on both nitrogen and oxygen atoms. To achieve greater stability, it is crucial to minimize these charges by converting lone pairs into bonds.
The formal charges on atoms can be minimized by converting a lone pair of the oxygen atom into a new N – O bond with the nitrogen atom.
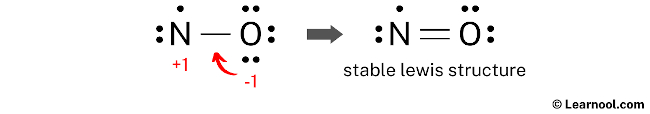
Final structure
The final structure of NO involves a nitrogen atom and an oxygen atom connected by a double covalent bond. In this arrangement, the molecule is a rare example of a stable radical, as the nitrogen atom serves as an exception to the octet rule by possessing an odd number of electrons—specifically, seven in its valence shell. The oxygen atom fulfills its octet by sharing two pairs of electrons and maintaining two lone pairs, while the nitrogen atom retains one lone pair and a single unpaired electron alongside the double bond. This configuration is the most stable because it results in formal charges of zero for both atoms, representing the most energetically favorable state for this neutral radical. Accordingly, this specific electronic distribution serves as the definitive and most accurate Lewis representation of nitric oxide.
Next: N2O Lewis structure
External video
- NO Lewis Structure – How to Draw the Lewis Structure for NO (Nitric Oxide) – YouTube • Wayne Breslyn
External links
- How can I draw the Lewis structure for NO? – Socratic
- NO Lewis Structure in 5 Steps (With Images) – Pediabay
- Why is it difficult to construct a Lewis structure for NO? – Quora
- Lewis Structures – Chemistry LibreTexts
- NO Lewis Structure – The Geoexchange
- Lewis structure of nitric oxide – Chemistry Stack Exchange
- Draw a Lewis structure for NO – Chegg
- NO lewis dot structure – Brainly
- Lewis Dot of Nitrogen Monoxide NO – Kent’s Chemistry
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
