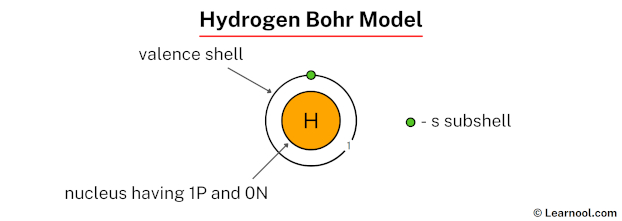
The Bohr model of hydrogen represents a simple atomic structure with a nucleus containing 1 proton and 0 neutrons, situated at the center.[1][2][3] Hydrogen has only one electron, which orbits the nucleus in a single electron shell. The model emphasizes the arrangement of these components, showcasing that hydrogen, being the simplest element, has a single proton and electron, with no neutrons in its nucleus.
To draw the hydrogen Bohr model, start by drawing the nucleus with 1 proton and 0 neutrons at the center. Then, sketch the first electron shell around the nucleus. Place the single electron in this shell, which is the only one required for hydrogen, and mark it clearly to indicate its position. The shell is labeled as K-shell, representing the only electron shell in hydrogen.
Steps
Write protons, neutrons, and electrons of hydrogen atom
Hydrogen has 1 proton, 0 neutron, and 1 electron.
Learn how to find: Hydrogen protons neutrons electrons
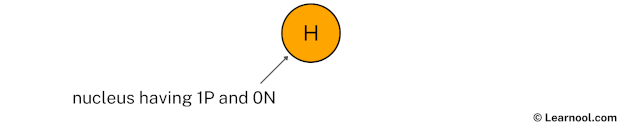
Draw nucleus of hydrogen atom
The nucleus of a hydrogen atom contains 1 proton and 0 neutron. So draw the nucleus of hydrogen atom as follows:
Now in the next step, draw the 1st electron shell and start marking electrons.
Draw 1st electron shell
Remember that we have a total of 1 electron.
The 1st electron shell (containing s subshell) can hold up to a maximum of 2 electrons. So draw the 1st electron shell as follows:
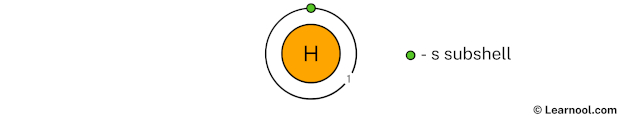
In the above image, 1 represents the 1st electron shell that contains 1s subshell. And the green color represents the number of electrons in that subshell. This means that the 1st electron shell has a total of 1 electron.
That’s it! This is the final Bohr model of hydrogen atom as we have only 1 electron and that is marked in the 1st electron shell.
Next: Helium Bohr model
Related
More topics
References
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
