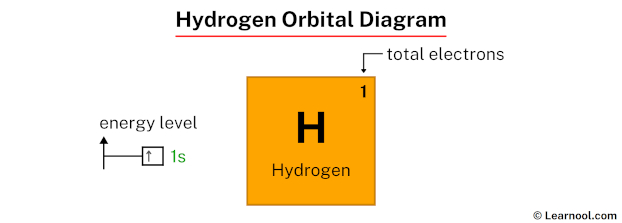
In the hydrogen orbital diagram, the 1s subshell contains a single electron.
To draw the hydrogen orbital diagram, start by determining the number of electrons from the periodic table. Next, note the electron configuration for reference, and follow the three essential rules: Aufbau principle, Pauli exclusion principle, and Hund’s rule. This ensures an accurate representation of hydrogen’s orbital arrangement.
Steps
Find electrons
To determine the number of electrons in a hydrogen atom, refer to its atomic number on the periodic table. Given that the atomic number of hydrogen is 1, it possesses a single electron.
Write electron configuration
The electron configuration of hydrogen is 1s1.
Now in the next step, start drawing the orbital diagram for hydrogen.
Draw orbital diagram
Before drawing the orbital diagram, you should know the three general rules.
- Aufbau principle – electrons are first filled in lowest energy orbital and then in higher energy orbital
- Pauli exclusion principle – two electrons with the same spin can not occupy the same orbital
- Hund’s rule – each orbital should be first filled with one electron before being paired with a second electron
Also, you should know the number of orbitals in each subshell.
We can calculate the number of orbitals in each subshell using the formula: 2ℓ + 1
Where, ℓ = azimuthal quantum number of the subshell
For s subshell, ℓ = 0
For p subshell, ℓ = 1
For d subshell, ℓ = 2
For f subshell, ℓ = 3
So each s subshell has one orbital, each p subshell has three orbitals, each d subshell has five orbitals, and each f subshell has seven orbitals.
Now start to draw!
As mentioned above, the electron configuration of hydrogen is 1s1. Hence, draw the blank orbital diagram of hydrogen up to 1s subshell as follows:
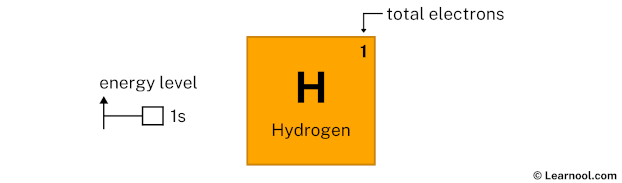
In the above orbital diagram, the box represents an orbital. Each orbital has a capacity of two electrons. And the arrows (↑↓) are drawn inside the box to represent electrons.
Now 1s1 indicates that the 1s subshell has 1 electron. So draw one arrow in the 1s box showing one electron as follows:
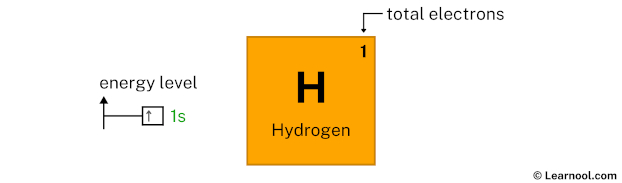
That’s it! This is the final orbital diagram of hydrogen as we have used all electrons.
Next: Helium orbital diagram
Related
More topics
External links
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
