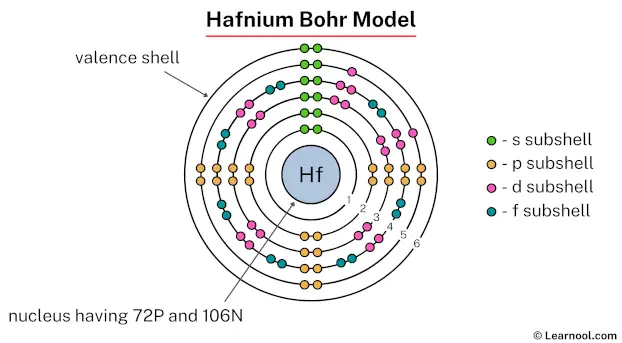
The hafnium Bohr model depicts a nucleus with 72 protons and 106 neutrons. Surrounding this nucleus are six electron shells, holding a total of 72 electrons.
To draw the hafnium Bohr model, represent the 72 protons, 106 neutrons, and 72 electrons. Start by illustrating the nucleus, and then draw the six electron shells. The first five shells should contain 2, 8, 18, 32, and 10 electrons, respectively, while the sixth shell holds the remaining 2 electrons.
Steps
Write protons, neutrons, and electrons of hafnium atom
Hafnium has 72 protons, 106 neutrons, and 72 electrons.
Learn how to find: Hafnium protons neutrons electrons
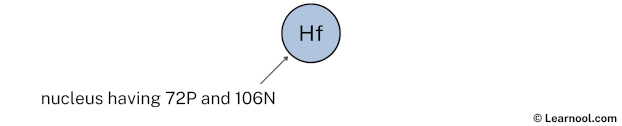
Draw nucleus of hafnium atom
The nucleus of a hafnium atom contains 72 protons and 106 neutrons. So draw the nucleus of hafnium atom as follows:
Now in the next step, draw the 1st electron shell and start marking electrons.
Draw 1st electron shell
Remember that we have a total of 72 electrons.
The 1st electron shell (containing s subshell) can hold up to a maximum of 2 electrons. So draw the 1st electron shell as follows:
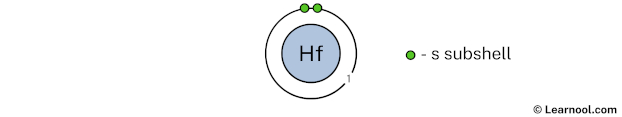
In the above image, 1 represents the 1st electron shell that contains 1s subshell. And the green color represents the number of electrons in that subshell. This means that the 1st electron shell has a total of 2 electrons.
Since we have already used 2 electrons in the 1st electron shell, now we have 72 – 2 = 70 electrons left. So in the next step, we have to draw the 2nd electron shell.
Draw 2nd electron shell
The 2nd electron shell (containing s subshell and p subshell) can hold up to a maximum of 8 electrons. So draw the 2nd electron shell as follows:
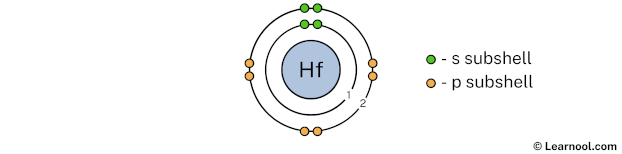
In the above image, 2 represents the 2nd electron shell that contains 2s and 2p subshells. And the green and orange color represents the number of electrons in that subshell. This means that the 2nd electron shell has a total of 8 electrons.
Now we have already used 10 electrons in 1st and 2nd electron shells, so we have 72 – 10 = 62 electrons left. So in the next step, we have to draw the 3rd electron shell.
Draw 3rd electron shell
The 3rd electron shell (containing s subshell, p subshell, and d subshell) can hold up to a maximum of 18 electrons. So draw the 3rd electron shell as follows:
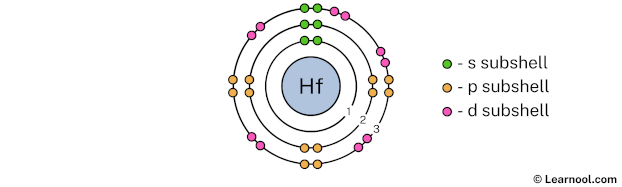
In the above image, 3 represents the 3rd electron shell that contains 3s, 3p, and 3d subshells. And the green, orange, and pink color represents the number of electrons in that subshell. This means that the 3rd electron shell has a total of 18 electrons.
Now we have already used 28 electrons in 1st, 2nd, and 3rd electron shells, so we have 72 – 28 = 44 electrons left. So in the next step, we have to draw the 4th electron shell.
Draw 4th electron shell
The 4th electron shell (containing s subshell, p subshell, d subshell, and f subshell) can hold up to a maximum of 32 electrons. So draw the 4th electron shell as follows:
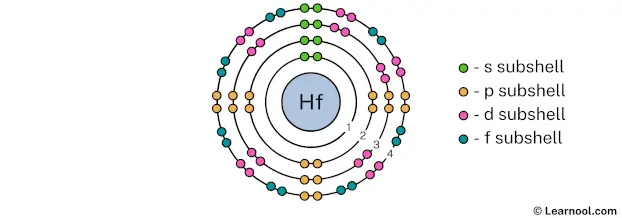
In the above image, 4 represents the 4th electron shell that contains 4s, 4p, 4d, and 4f subshells. And the green, orange, pink, and blue color represents the number of electrons in that subshell. This means that the 4th electron shell has a total of 32 electrons.
Now we have already used 60 electrons in 1st, 2nd, 3rd, and 4th electron shells, so we have 72 – 60 = 12 electrons left. So in the next step, we have to draw the 5th electron shell.
Draw 5th electron shell
The 5th electron shell can hold up to a maximum of 50 electrons. So draw the 5th electron shell as follows:
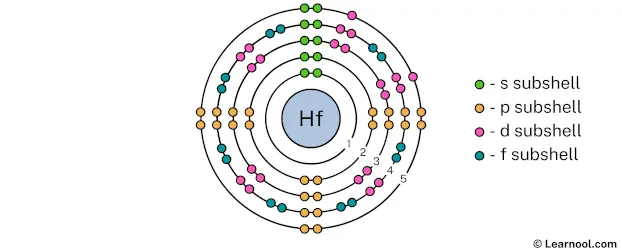
In the above image, 5 represents the 5th electron shell that contains 5s, 5p, and 5d subshells. And the green, orange, and pink color represents the number of electrons in that subshell. This means that the 5th electron shell has a total of 10 electrons.
The 5th electron shell contains only 5s, 5p, and 5d subshells, and not a 5f subshell. This is because according to the aufbau principle, the 6s subshell is filled first and then 4f, 5d, 6p… and so on.
Now we have already used 70 electrons in 1st, 2nd, 3rd, 4th, and 5th electron shells, so we have 72 – 70 = 2 electrons left. So in the next step, we have to draw the 6th electron shell.
Draw 6th electron shell
The 6th electron shell can hold up to a maximum of 72 electrons. So draw the 6th electron shell as follows:
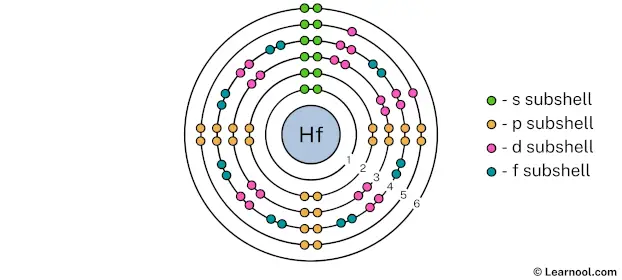
In the above image, 6 represents the 6th electron shell that contains 6s subshell. And the green color represents the number of electrons in that subshell. This means that the 6th electron shell has a total of 2 electrons.
That’s it! This is the final Bohr model of hafnium atom as we have used all 72 electrons: 2 electrons in the 1st electron shell, 8 electrons in the 2nd electron shell, 18 electrons in the 3rd electron shell, 32 electrons in the 4th electron shell, 10 electrons in the 5th electron shell, and 2 electrons in the 6th electron shell.
Next: Tantalum Bohr model
Related
More topics
External links
- File:72 halfnium (Hf) enhanced Bohr model.png – Wikimedia Commons
- What is the Bohr model for Hafnium? – Topblogtenz
- Chemical Elements.com – Hafnium (Hf) – Chemical Elements.com
- Hafnium (Hf) – Periodic Table – ChemicalAid
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
