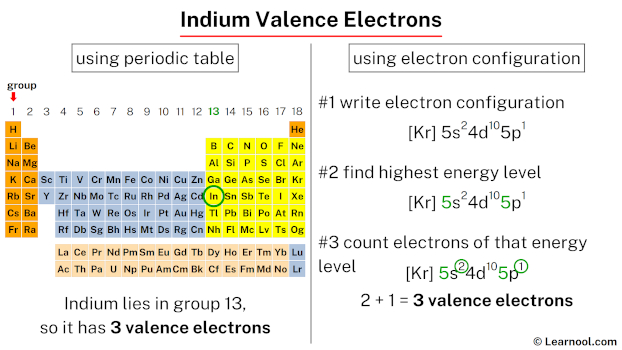
Indium is classified as a post-transition metal and holds 3 valence electrons. To determine the valence electrons for indium, one method is to directly locate its position on the periodic table – which is group 13 – or to use its electron configuration as another method.
Methods
Using periodic table
Get the periodic table having the chemical elements marked on it as mentioned above.
Now mark the location of indium on the periodic table.
Next, mark the group number of indium on the periodic table.
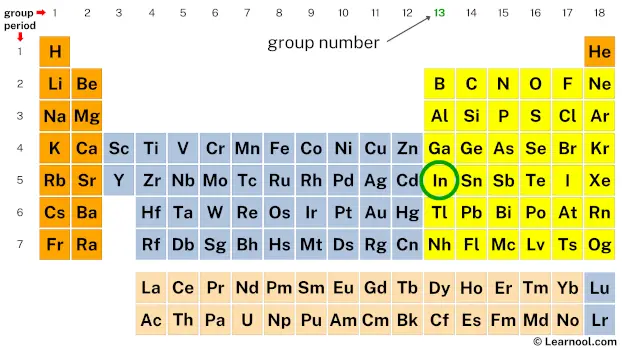
The valence electrons of each main-group element can be determined by the column in which it is located.
(i.e., all group 1 elements have 1 valence electron, all group 2 elements have 2 valence electrons, skip the transition metals… then, all group 13 elements have 3 valence electrons, all group 14 elements have 4 valence electrons, and so on up to group 18 elements)
Since indium is in group 13, it has 3 valence electrons.
Using electron configuration
- First, write electron configuration of indium
The electron configuration of indium is [Kr] 5s2 4d10 5p1.
- Second, find highest energy level in electron configuration
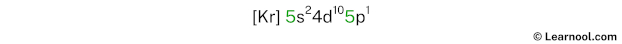
In the above electron configuration, the highest energy level (5) is marked with green color.
- Finally, count electrons of that energy level
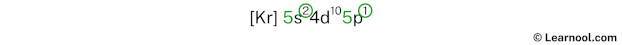
The 5th energy level contains 5s and 5p subshells. There are 2 electrons in the 5s subshell and 1 electron in the 5p subshell. So indium has a total of 2 + 1 = 3 valence electrons.
Next: Tin valence electrons
Related
More topics
External links
- https://homework.study.com/explanation/how-many-valence-shell-electrons-does-an-atom-of-indium-have.html
- https://materials.gelsonluz.com/2019/08/valence-electrons-in-indium-in-facts.html
- https://brainly.com/question/9489307
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
