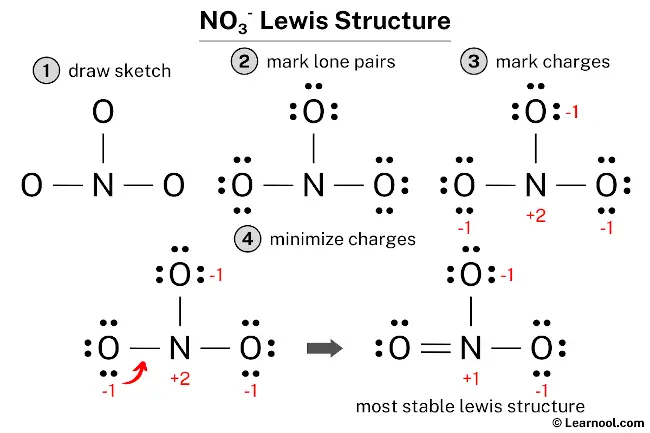
The NO3– Lewis structure represents the nitrate ion, which consists of one nitrogen atom and three oxygen atoms. The central nitrogen atom forms one double bond and two single bonds with the three surrounding oxygen atoms. The oxygen atom involved in the double bond has two lone pairs, while each of the two oxygen atoms involved in single bonds has three lone pairs. The nitrogen atom in the NO3– Lewis structure carries a positive charge of +1, while the two oxygen atoms attached to it through single bonds each carry a negative charge of -1.
To draw the NO3– Lewis structure, begin by sketching a rough skeleton structure of the molecule. Identify any lone pairs of electrons on the atoms and incorporate them into the structure. Calculate the formal charges for each atom and minimize them by converting lone pairs to double or triple bonds to achieve a stable Lewis structure. Repeat this process if needed until all charges are minimized, ensuring that the octet rule is satisfied for both the central atom and the surrounding atoms. By following these simple steps, an accurate and complete NO3– Lewis structure can be drawn.
Steps
Sketch the structure
The first step in drawing the NO3– Lewis structure is to determine the total number of valence electrons present in the molecule. This can be calculated by multiplying the valence electrons of each atom. Nitrogen, belonging to group 15 of the periodic table, has five valence electrons, while oxygen, belonging to group 16, has six valence electrons. NO3– consists of one nitrogen atom and three oxygen atoms, so the total valence electrons can be calculated as follows: 5 valence electrons for the nitrogen atom, plus 6 valence electrons for each of the three oxygen atoms, plus 1 additional electron due to the negative charge. This results in a total of 24 valence electrons.
Learn how to find: Nitrogen valence electrons and Oxygen valence electrons
The second step in drawing the NO3– Lewis structure involves determining the total number of electron pairs, which can be calculated from the total number of valence electrons in the molecule. In the case of NO3–, which has a total of 24 valence electrons, the number of electron pairs is found by dividing the total valence electrons by 2, resulting in 12 electron pairs.
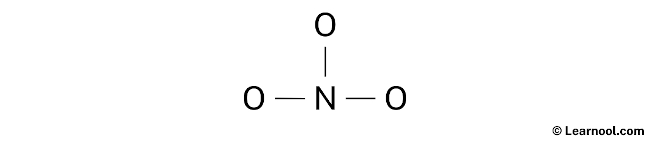
The third step is to identify the central atom. The least electronegative atom is typically placed at the center. In this case, nitrogen is less electronegative than oxygen, so it is assumed to be the central atom. Therefore, nitrogen is placed in the center, and oxygen atoms are positioned on either side. Finally, a rough sketch of the structure can be drawn. With the central atom and its surrounding atoms determined, proceed to draw a rough sketch for the NO3– Lewis structure.
Indicate lone pair
Once the rough sketch for the NO3– Lewis structure has been drawn, the next step is to identify the lone pairs. With a total of 12 electron pairs, it is important to note that three N – O bonds have already been shown, indicating the utilization of three electron pairs. As a result, the remaining nine electron pairs should be marked as lone pairs on the sketch to ensure an accurate representation of the electron distribution in the NO3– molecule.
It’s important to keep in mind that both nitrogen and oxygen are period 2 elements, which means that they can only accommodate a maximum of 8 electrons in their outermost shell.
It is recommended to begin marking the lone pairs from the outer atoms, which in this case are the oxygen atoms. Each oxygen atom will receive three lone pairs, while nitrogen will receive zero lone pairs since all nine electron pairs are already accounted for. The lone pairs can then be marked on the sketch accordingly to their respective atoms.
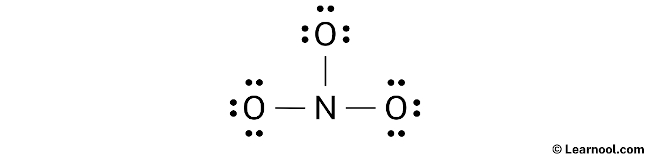
Assign formal charge
To determine the formal charges of the atoms in the NO3– ion, use the formula: Formal charge = valence electrons – nonbonding electrons – ½ bonding electrons. For the nitrogen atom, which has five valence electrons, zero nonbonding electrons, and six bonding electrons, the formal charge is +2. On the other hand, for each of the oxygen atoms, which have six valence electrons, six nonbonding electrons, and two bonding electrons, the formal charge is -1.
Since both nitrogen and oxygen atoms in NO3– have formal charges, it is important to mark these charges on the sketch.
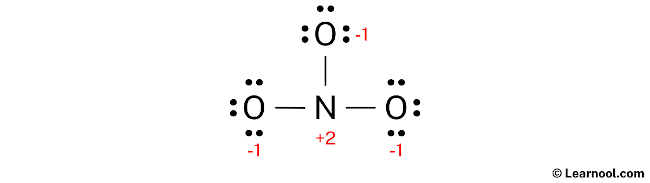
Since both the nitrogen and oxygen atoms in the NO3– ion have formal charges, the current Lewis structure is unstable. To achieve stability, lone pairs can be converted to bonds in order to reduce the charges. For instance, it is possible for one of the oxygen atoms to donate a lone pair, forming a double bond with the central nitrogen atom. This results in a reduction of formal charges on both nitrogen and oxygen atoms, leading to a more stable Lewis structure for NO3–.
Minimize formal charge
Reduce formal charges on nitrogen and oxygen atoms by converting a lone pair from one of the oxygen atoms into a new N – O bond with the nitrogen atom.
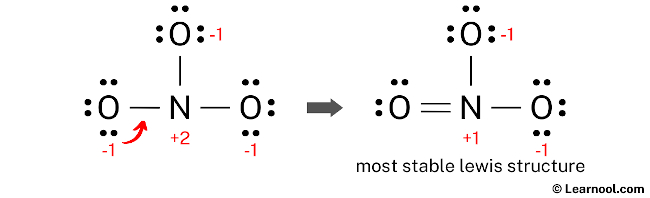
The NO3– Lewis structure now satisfies the octet rule as the central nitrogen atom and the three surrounding oxygen atoms have eight valence electrons each. However, there are still formal charges on the atoms. The two oxygen atoms have a negative (-1) charge, while the nitrogen atom has a positive (+1) charge. This is acceptable because the Lewis structure with a negative charge on the most electronegative atom is the most stable. And in this case, oxygen is the most electronegative element. This Lewis structure is more stable than the previous ones. Therefore, it can be considered as the most stable Lewis structure for NO3–.
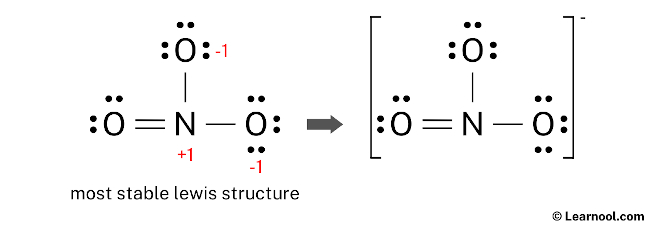
It is important to note that NO3– carries a negative (-1) charge, which should be indicated on the Lewis structure by enclosing the entire structure in brackets and including the charge as a superscript outside the brackets.
Next: O3 Lewis structure
External video
- NO3- Lewis Structure: How to Draw the Lewis Structure for NO3- – Wayne Breslyn
External links
- Lewis Structure for NO3- (Nitrate Ion) – The University of Maryland
- Lewis Dot of Nitrate Ion NO3- – Kent’s Chemistry
- Drawing the Lewis Structure for NO3- (Nitrate Ion) – The Geoexchange
- Lewis Structure for NO3- – Laurence Lavelle
- What is the Lewis dot structure for NO3-1? – Quora
- Draw the Lewis structures for three resonance forms of the nitrate ion, NO−3 – Brainly
- What are the 3 Lewis structures for NO3–? – Socratic
- What is the total number of valence electrons in the Lewis structure of the nitrate ion, NO3–? – Homework.Study.com
- Drawing the Lewis structure of a nitrate ion, NO3- – Reddit
- Draw the Lewis structures for the nitrate ion, NO3 – Chegg
- How is the Nitrate Ion (NO3) formed? – Chemistry Stack Exchange
- Lewis structure for nitrate, NO3 – An Introduction to Chemistry
- lewis structure no3- – Quizlet
- Formal Charge of Nitrate (NO3-) – Chemistry Learner
- lewis structure of no3- – Chemical Forums
- Confusion over the Lewis structure of the Nitrate ion,NO3- – The Student Room
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
