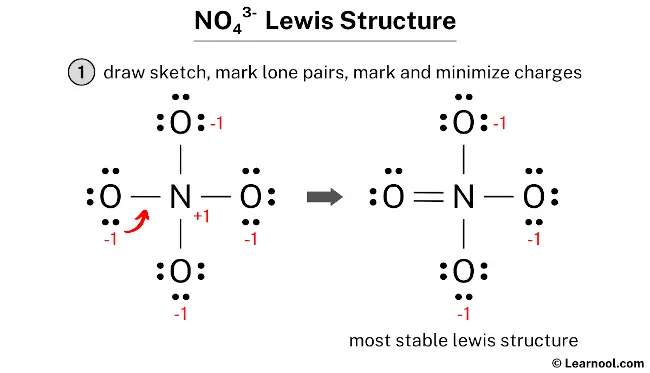
The NO43- Lewis structure represents the molecular arrangement for orthonitrate, a compound composed of one nitrogen atom and four oxygen atoms. In the structure, the nitrogen atom is surrounded by one double bond and three single bonds, with four oxygen atoms attached to it. Among the oxygen atoms, the one with a double bond contains two lone pairs, while the three oxygen atoms with single bonds each have three lone pairs. These three oxygen atoms with single bonds also carry a negative (-1) charge.
To draw the NO43- Lewis structure, begin by sketching a rough skeleton structure of the molecule. Once the rough structure is drawn, identify any lone pairs of electrons on the atoms and indicate them on the structure. Next, calculate the formal charges for each atom and minimize them by adjusting lone pairs to form double or triple bonds as needed, aiming to achieve a stable Lewis structure with minimized charges. If necessary, repeat the process until all charges are minimized, ensuring that all atoms satisfy the octet rule. By following these simple steps, one can accurately draw the NO43- Lewis structure.
Steps
Sketch the structure
The first step in drawing the NO43- Lewis structure is to determine the total number of valence electrons. The total valence electrons in the NO43- molecule can be calculated by multiplying the valence electrons of each atom. Nitrogen, a member of group 15, possesses five valence electrons, while oxygen, belonging to group 16, has six valence electrons. With one nitrogen atom and three oxygen atoms in NO43-, the nitrogen atom contributes 5 valence electrons, and each oxygen atom contributes 6 valence electrons, resulting in a total of 24. Taking into account the -3 charge on NO43-, an additional three electrons need to be included. Therefore, the total number of valence electrons for NO43- is 5 + 24 + 3 = 32.
Learn how to find: Nitrogen valence electrons and Oxygen valence electrons
After determining the total number of valence electrons, the next step in constructing the Lewis structure of NO43- is to calculate the total electron pairs. In the case of NO43-, which has a total of 32 valence electrons, the electron pairs can be determined by dividing 32 by 2. This calculation results in a total of 16 electron pairs for the NO43- molecule.
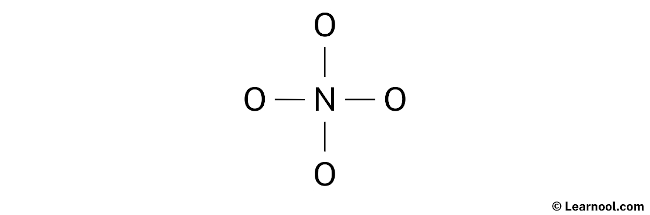
The next step involves determining the central atom in the NO43- Lewis structure. To do this, the atom with the least electronegativity is chosen to occupy the central position. In the case of NO43-, nitrogen is identified as having lower electronegativity compared to oxygen. Therefore, nitrogen is selected as the central atom, while the oxygen atoms are positioned on either side. Finally, the rough sketch of the NO43- Lewis structure can be drawn based on this arrangement.
Indicate lone pair
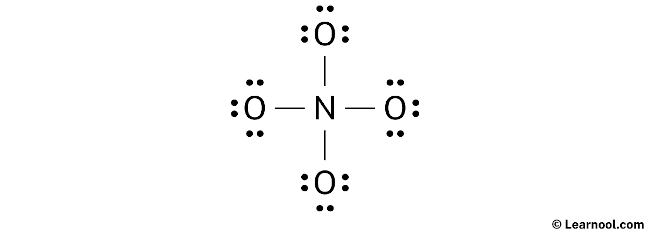
After sketching the rough structure, the next step in drawing the NO43- Lewis structure is to indicate the lone pairs. In total, there are 16 electron pairs. With four N – O bonds already marked on the sketch, which accounts for eight electron pairs, the remaining 12 electron pairs should be marked as lone pairs.
It’s worth noting that nitrogen and oxygen belong to the second period of the periodic table, which means they have a maximum capacity of 8 electrons in their outermost shells.
The process of marking lone pairs starts from the outer atoms. In the case of NO43-, the molecule contains four oxygen atoms, which are the outer atoms. Each oxygen atom will have three lone pairs assigned to it. However, for nitrogen, no lone pair will be marked since all twelve electron pairs are already accounted for. Proceed to mark the lone pairs on the sketch accordingly.
Assign formal charge
In order to determine the formal charges on the atoms within NO43-, use the following formula: Formal charge = valence electrons – nonbonding electrons – ½ bonding electrons. Applying this formula, the formal charge on the nitrogen atom is calculated as 5 – 0 – ½ (8) = +1, while each oxygen atom carries a formal charge of 6 – 6 – ½ (2) = -1.
Both nitrogen and oxygen exhibit formal charges, as determined through calculations, and it is important to mark these charges on the respective atoms in the sketch.
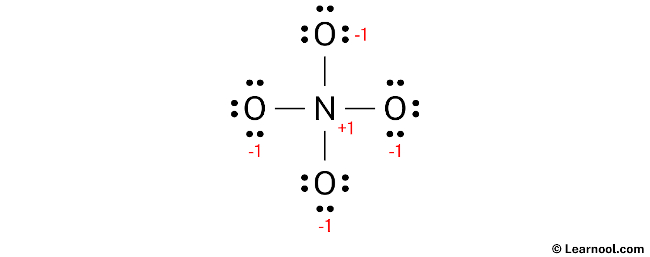
The existing structure of NO43- cannot be considered as a stable Lewis structure since both the nitrogen and oxygen atoms exhibit formal charges. It is not a suitable final Lewis structure for NO43- because NO43- is an ion with a net negative charge of -3, whereas in the current structure, each oxygen atom carries a charge of -1. In order to achieve a net formal charge of -3, it is essential to decrease these charges by converting lone pairs into bonds.
Minimize formal charge
In order to achieve a net negative charge of -3 in NO43-, an exception can be made for nitrogen, allowing it to accommodate more than 8 electrons in its outermost shell. With this approach, to reduce the formal charges on atoms, a lone pair from one of the oxygen atoms can be converted into a new N – O bond with the nitrogen atom.
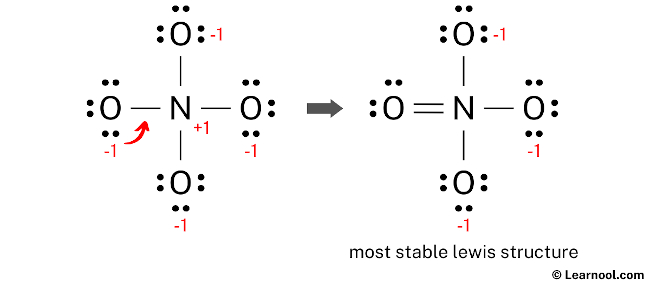
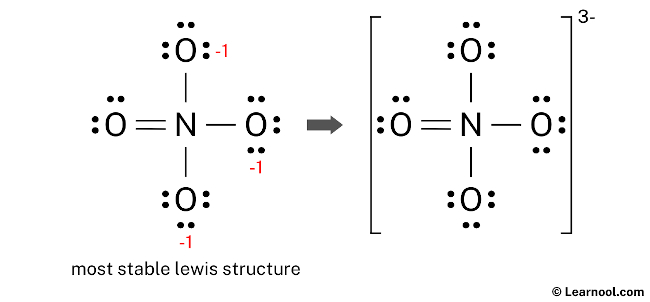
The final structure features the central atom (nitrogen) forming an octet, while the four outside oxygen atoms also form an octet. As a result, the structure satisfies the octet rule. However, there remains a negative charge (-1) on the three oxygen atoms. This is acceptable since the best Lewis structure is the one with a negative charge on the most electronegative atom, which, in this case, is oxygen. The above structure exhibits greater stability compared to the previous structures. Therefore, it represents the most stable Lewis structure for NO43-. Since NO43- carries a negative charge (-3), it is important to indicate this charge on the Lewis structure by drawing brackets.
Next: CH3CH2NH2 Lewis structure
External links
- What’s the Lewis structure of NO4 -3? – Quora
- Draw the Lewis structure for NO43- – Homework.Study.com
- Draw a Lewis structure for NO4-3 then label the formal charges on each atom and finally propose a structure to minimize the formal charge – Brainly
- Draw a Lewis structure for NO4-3 then label the formal charges on each atom and finally propose a structure to minimize the formal charge – Chegg
- Draw the Lewis Structure for NO4 3- (as well as any resonance structures) and offer an explanation why the NO4 3- anion is unstable – Numerade
- Whats the Lewis structure of NO4 -3? – ECHEMI
- Orthonitrate – Wikipedia
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
