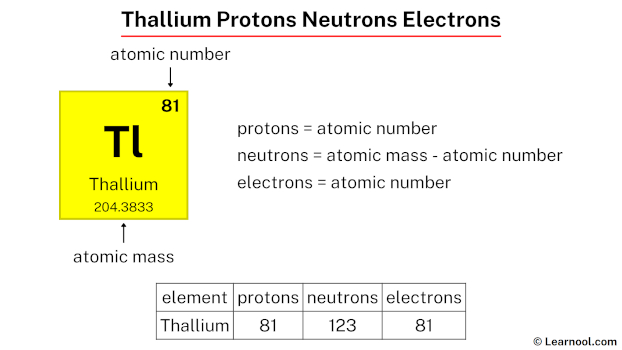
Thallium, a highly toxic metal with various industrial applications, consists of 81 protons, 123 neutrons, and 81 electrons.
Thallium protons
- Protons = atomic number
From the periodic table, find the atomic number of thallium.
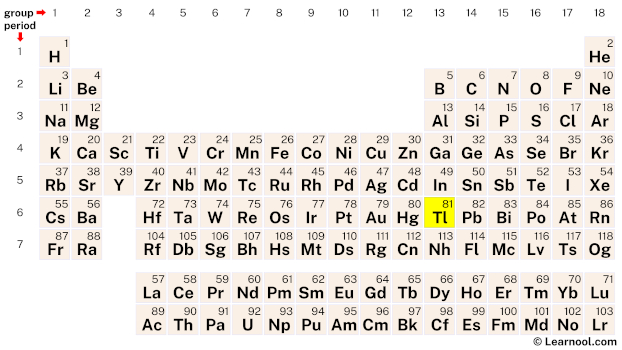
The atomic number of thallium is 81. Hence, thallium has a total of 81 protons.
Thallium neutrons
- Neutrons = atomic mass – atomic number
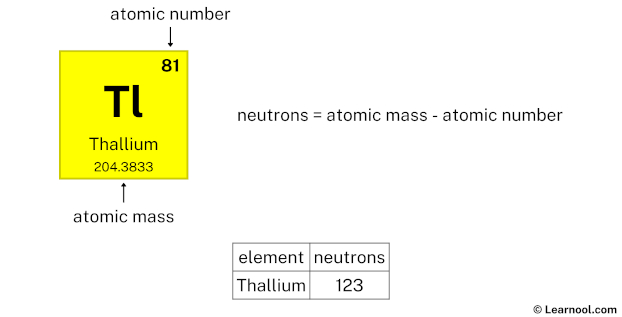
The atomic mass of thallium is 204.3833, so we’ll take the roundup value as 204. And the atomic number of thallium is 81.
Subtract the atomic number (81) from the atomic mass (204). Hence, thallium has a total of 204 – 81 = 123 neutrons.
Thallium electrons
- Electrons = atomic number
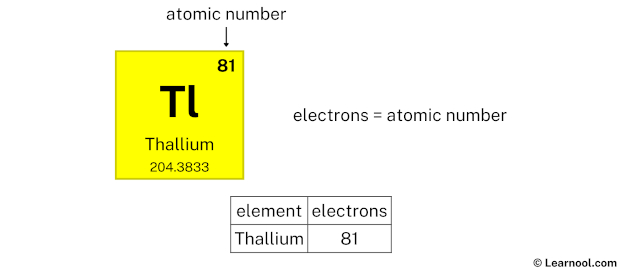
The atomic number of thallium is 81. Hence, thallium has a total of 81 electrons.
Next: Lead protons neutrons electrons
Related
More topics
External links
- https://material-properties.org/thallium-protons-neutrons-electrons-electron-configuration/
- https://valenceelectrons.com/thallium-protons-neutrons-electrons/
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
