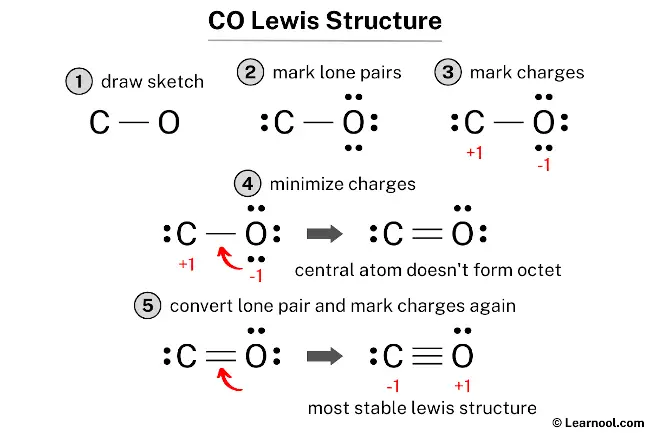
The CO Lewis structure illustrates the molecular arrangement of carbon monoxide, a molecule composed of one carbon atom and one oxygen atom. In the CO Lewis structure, there is a triple bond between the carbon and oxygen atoms, with each atom possessing one lone pair. The carbon atom carries a negative (-1) charge, while the oxygen atom has a positive (+1) charge.
To draw a CO Lewis structure, start by sketching a rough structure of the molecule and indicating the positions of any lone pairs on the atoms. Formal charges on the atoms should be marked if necessary. Then, convert the lone pairs of atoms to minimize the formal charges. Repeat this step until all charges are minimized. Finally, check whether both the central atom and the surrounding atoms satisfy the octet rule. If any atom does not form an octet, convert a lone pair to a chemical bond and reassess the charges. By following these steps, an accurate and complete CO Lewis structure can be drawn.
Steps
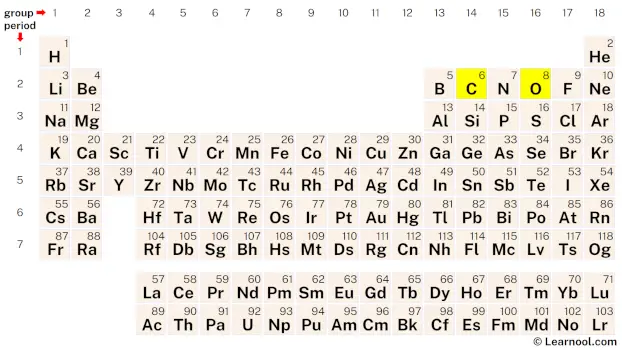
Sketch the structure
To start drawing the CO Lewis structure, the first step is to determine the total number of valence electrons in the molecule. The total valence electrons in the molecule can be calculated by multiplying the valence electrons of each atom. Carbon, which belongs to group 14 of the periodic table, has four valence electrons, while oxygen, belonging to group 16, has six valence electrons. As CO contains one carbon atom and one oxygen atom, the carbon atom contributes 4 valence electrons, and the oxygen atom contributes 6 valence electrons, resulting in a total of 4 + 6, which equals 10 valence electrons.
Learn how to find: Carbon valence electrons and Oxygen valence electrons
The second step in drawing the CO Lewis structure is to determine the total number of electron pairs. This can be calculated by dividing the total number of valence electrons, which is 10 for CO, by two. Therefore, the total number of electron pairs in CO is 5.
After the total electron pairs have been calculated, the next step in drawing the CO Lewis structure is to determine the central atom. This is done by placing the least electronegative atom at the center. In the case of CO, carbon is less electronegative than oxygen, so it is assumed that the central atom is carbon. Once the central atom has been determined, the rough sketch can be drawn.
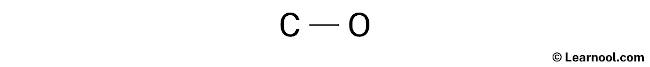
Indicate lone pair
After drawing the rough sketch of the molecule, the next step is to indicate the lone pairs. As calculated earlier, there are a total of 5 electron pairs in CO. One C – O bond, utilizing one electron pair, has already been marked in the sketch, leaving four electron pairs to be marked as lone pairs.
It is important to keep in mind that carbon and oxygen, both being period 2 elements, cannot accommodate more than 8 electrons in their valence shell.
To indicate the lone pairs, it is recommended to begin with the outer atoms. In the case of CO, oxygen is the outer atom. Therefore, it has three lone pairs around it, whereas carbon has only one lone pair that should also be marked on the sketch. The lone pairs can be indicated on the sketch in the following manner:
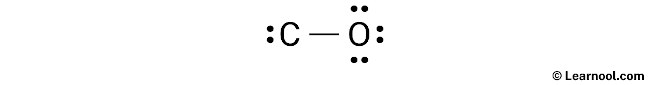
Assign formal charge
To determine the formal charges on the atoms in the CO molecule, the following formula can be used: Formal charge = valence electrons – nonbonding electrons – ½ bonding electrons. Applying this formula, the formal charge on the carbon atom is calculated as +1, since it has 4 valence electrons, 2 nonbonding electrons, and 2 bonding electrons. Similarly, the formal charge on the oxygen atom is calculated as -1, as it has 6 valence electrons, 6 nonbonding electrons, and 2 bonding electrons.
After calculating the formal charges, it is important to mark the charges on the sketch to indicate that both atoms have charges.
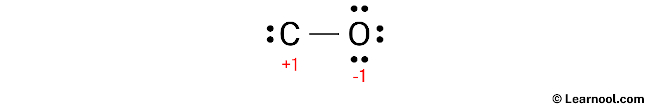
Since both the carbon and oxygen atoms in the structure have charges, the current Lewis structure is not stable. To achieve a stable structure, it is necessary to reduce the charges by converting lone pairs to bonds.
Minimize formal charge
To minimize the formal charges, one of the lone pairs on the oxygen atom should be converted into a new C – O bond with the carbon atom.
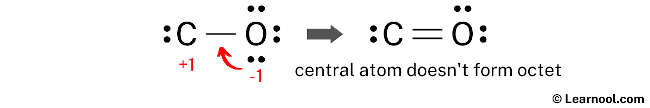
The above structure does not satisfy the octet rule, as the central atom (carbon) does not form an octet. Therefore, it is recommended to convert another lone pair to a bond and recalculate the formal charges to attain a more stable Lewis structure.
Convert lone pair and recalculate formal charge

Once the lone pair of oxygen is converted into a new C – O bond with the carbon atom, it is crucial to recalculate the formal charges on each atom to ensure that no charges remain. This will help determine whether the Lewis structure is stable or not.
Use the formula used earlier, to calculate the new formal charges: Formal charge = valence electrons – nonbonding electrons – ½ bonding electrons. For the carbon atom, formal charge = 4 – 2 – ½ (6) = -1. For the oxygen atom, formal charge = 6 – 2 – ½ (6) = +1.
As both atoms still have charges, mark them on the sketch again to indicate the charges.
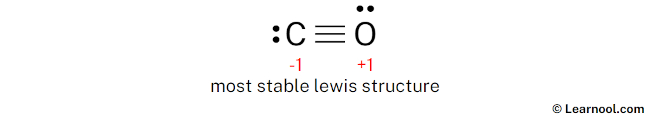
The structure now satisfies the octet rule, with the central atom (carbon) and the outside atom (oxygen) forming octets. However, charges still remain on the atoms, and converting a lone pair to a bond is not an option since carbon cannot exceed 8 electrons in its last shell. The formal charges on the atoms are closer to zero, and this structure is more stable than previous ones. Hence, this is the most stable Lewis structure of CO.
Next: CH4 Lewis structure
External video
- How to Draw the Lewis Dot Diagram for Carbon monoxide (CO) – Wayne Breslyn
External links
- Why does Lewis dot structure of CO not have formal charges close to 0? – Chemistry Stack Exchange
- Draw the Lewis structure for CO – Homework.Study.com
- What is the Lewis Structure of CO? – Quora
- CO Lewis Structure in 5 Steps (With Images) – Pediabay
- Lewis Structure for CO – The University of Maryland
- How can I draw the Lewis structure for CO? – Socratic
- a bit puzzled by carbon monoxide lewis structure – Reddit
- How to draw the Lewis structure of CO – ChemicalBook
- CO Lewis Structure – Laurence Lavelle
- CO Lewis Structure – Pinterest
- Lewis Structure of CO2 [with video and free study guide] – AceOrganicChem
- Chemical Bonding: CO Lewis Structure – The Geoexchange
- Lewis Dot of Carbon Monoxide CO – Kent’s Chemistry
- Draw the Lewis structure of CO. Include lone pairs – Chegg
- What is the Lewisian structure of CO? – FAQ – Guidechem
- The Lewis structure for carbon monoxide is : C = O – Brainly
- Give the Lewis dot structure for the molecules carbon monoxide (CO) – Pearson
Deep
Learnool.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
